1. Background
Heat is reported to have detrimental effects on the functioning of patients with MS (1-6). Our previous studies have shown temporary decline of both cognitive and physical functioning after short-term heat exposure in a Finnish sauna (1, 2). Assessment of such negative effects of heat can be laborious because it requires arrangements of heat exposure and versatile measurement tools as well as repeated testing sessions. Thus, simple measurement tools are used to evaluate the effects of heat in patients with MS.
Several different impairment scales have been created for use in MS (7-10). Of these, the expanded disability scale (EDSS) has more or less become the evaluation standard. However, the EDSS is known to be insensitive to changes in arm function and cognitive abilities, and is greatly influenced by the limitations of the leg function. The multiple sclerosis functional composite (MSFC) is a multidimensional, standardized and quantitative assessment instrument that was developed to account for different aspects of functioning in the clinical trials of MS (7, 11). Compared with the EDSS, the MSFC has the methodological advantage of measuring physical as well as cognitive disability, producing scores for three individual measures as well as a total composite score (8). The MSFC is relatively easy to administer and yet a versatile measure with total execution time of approximately 20 - 30 minutes. It has been used predominantly as an outcome measure in clinical drug trials; less is known about its feasibility in other types of experimental studies of MS (12).
2. Objectives
The aim of the present study was to evaluate whether the MSFC is able to detect the effects of heat on functioning in patients with MS.
3. Patients and Methods
3.1. Subjects
The study was conducted in Masku neurological rehabilitation center (Finland), and the study group used in the current analysis was the same as our previous studies (1, 2). In total, 22 individuals with MS and 19 healthy controls were included in the analysis. All the patients reported heat sensitivity and felt that heat induces or exacerbates their fatigue or worsens their functioning. The inclusion criteria were, confirmed MS diagnosis, relapsing-remitting or secondary progressive MS, a score of 0 to 5.5 on the expanded disability status scale (EDSS), and age between 20 and 55 years. Subjects were excluded from the study if they had a primary progressive disease course, relapse during the preceding month, cardiac disease, hypertension or significantly increased blood pressure (systolic pressure > 160 mmHg, diastolic > 95 mmHg) or other diseases likely to preclude sustained heat exposure, or if they showed signs of any other medical or mental condition precluding participation, and were diagnosed with a central nervous system disease other than MS. The HCs were matched with the MS group by gender, age, and education. The three last-mentioned exclusion criteria were also used in the screening for the HCs.
3.2. Procedure
The entire study procedure is described in details elsewhere (1, 2). The study was approved by the ethical committee of the local health care district and all study subjects provided written informed consent before participation. All the measures analyzed in the current study are known to be valid, reliable and widely used in the field of MS research. The participants were familiarized with the protocol the day before the actual screening. During the second day, the participants underwent three testing sessions: before the heat exposure (baseline), during or immediately after the exposure, and one hour after the exposure. The conditions as well as the food and liquid intake were controlled. The heat exposure took place in a Finnish sauna (for more information see Hamalainen et al., 2012 (1)) and lasted for approximately 45 minutes. During the assessments, the participants’ core temperature was also monitored. All the assessments were performed by the same assessor (AI).
3.3. Multiple Sclerosis Functional Composite
The MSFC is a multidimensional and reliable MS-specific measurement tool that includes measures of arm function (Nine Hole Peg Test, 9HPT), ambulation (Timed 25-Foot Timed Walk, TWT) and attention (Paced Auditory Serial Addition Test 3”version, PASAT-3) (13, 14).
The 9HPT is a measure of upper extremity (arm and hand) function (15). The test result is the time required to place nine pegs into nine holes and then remove them. Both hands are tested twice. In the present study the mean performance time for each hand was first calculated separately for each of the three time points. The variable used in the MSFC score was then determined by calculating the mean of these two measures (16). The test was practiced once with each hand before the actual screening.
Timed 25-Foot Timed Walk is a measure of lower extremity function / ambulation (16). During the test, the patient is directed to one end of a clearly marked 25-foot (7.62 m) course and instructed to walk 25 feet as quickly as possible. The execution time serves as the result. The use of assistive devices was permitted during the test. The task was carried out twice, and the variable used in the MSFC score is determined by calculating the mean of these two trials (16). In the present study, the task was practiced once before the actual screening.
The PASAT is a measure of cognitive function that specifically assesses auditory information processing speed, ability to focus and sustain attention and calculation ability (17). In the PASAT-3, the participants were instructed to listen to 61 single digits presented at three-second intervals. The subjects were instructed to add all consecutive two digits in a row and give the answer to the examiner. The test result used in the MSFC calculation formula is the number of correct answers. The intra- and inter-rater reliability of the Finnish PASAT-3-version is good, although the test is prone to considerable practice effects (13, 14). In the present study, the test was practiced twice, and the third trial served as the baseline performance. Two parallel versions of the test were used alternately.
In the MSFC, separate scores for the three individual measures (z-scores) as well as a total composite score (the MSFC-score) are computed (16). Deterioration in the MSFC score represents deterioration in overall functioning. The calculation formula used in the present study was as instructed in the MSFC Manual (16):
MSFC Score = [(Average (1/9HP-test) - Baseline Mean (1/9HP-Test) / Baseline SD (1/9HP-test) + [- (Average TWT – Baseline Mean TWT) / Baseline SD TWT] + (PASAT-3 - Baseline Mean PASAT-3) / Baseline SD PASAT 3]/Baseline SD PASAT-3] / 3.0
The z-scores of the three components of MSFC were standardized using the combined scores of the MS patients and the HCs.
3.4. Statistical Analyses
The differences between the study groups were analyzed with the χ2 test in terms of gender, and with the t-test in terms of age, education and mood. The Z scores and the MSFC scores were calculated for each subject separately as guided in the MSFC-manual (15). The Kolmogorov-Smirnov test was then used to determine whether the scores for each component of the MSFC were distributed normally. To evaluate the MSFC as a measure of heat sensitivity, the means of the MSFC scores were analyzed using repeated measures analysis of variance (group as a between‐subject factor, and measure point as a within-subject factor). The contrasts were carried out to determine the time point in which the performance changed between the two groups. All statistical analyses were performed using the SPSS 16.0 for Windows software.
4. Results
4.1. Variables
Twenty-four MS patients and 19 healthy controls (5 male and 14 female) entered the screening background examination. One patient was excluded because of primary progressive MS and another because of scores of more than three standard deviations from the mean, leaving 22 patients (5 males and 18 females) with MS for statistical evaluations (Table 1). The two groups were matched in terms of gender (P = 0.73), age (P = 0.56) and education (P = 0.27). The baseline mean core body temperature in both groups was 37.2°C/99.0°F (P = 0.93). The average increase in core temperature from the beginning to the end of the heat exposure was higher in the MS group than in the HC group (MS 0.5°C /32.9°F, HC 0.2°C /32.4°F, P = 0.00). The mean core body temperature did not differ between the groups at one-hour delay assessment (P = 0.69).
| Value | MS Subjects (n = 23), Mean (SD) | Healthy Controls (n = 19), Mean (SD) |
|---|---|---|
| Age, y | 42.3 (7.0) | 40.6 (10.9) |
| Education, y | 13.7 (2.8) | 14.7 (2.6) |
| Disease duration, y | 8.3 (5.0) | NA |
| EDSS | 2.9 (1.1) | ND |
| MSFC-score | -0.48 (0.79) | 0.58 (0.42) |
| TWT, s | 4.8 (0.9) | 3.3 (0.5) |
| 9HPT, s | 23.1 (5.2) | 16.9 (1.6) |
| PASAT-3 (total correct) | 49.5 (8.8) | 53.9 (6.3) |
Demographic Characteristics and Mean Scores of the Multiple Sclerosis Functional Composite and its Three Components at Baseline
4.2. Multiple Sclerosis Functional Composite
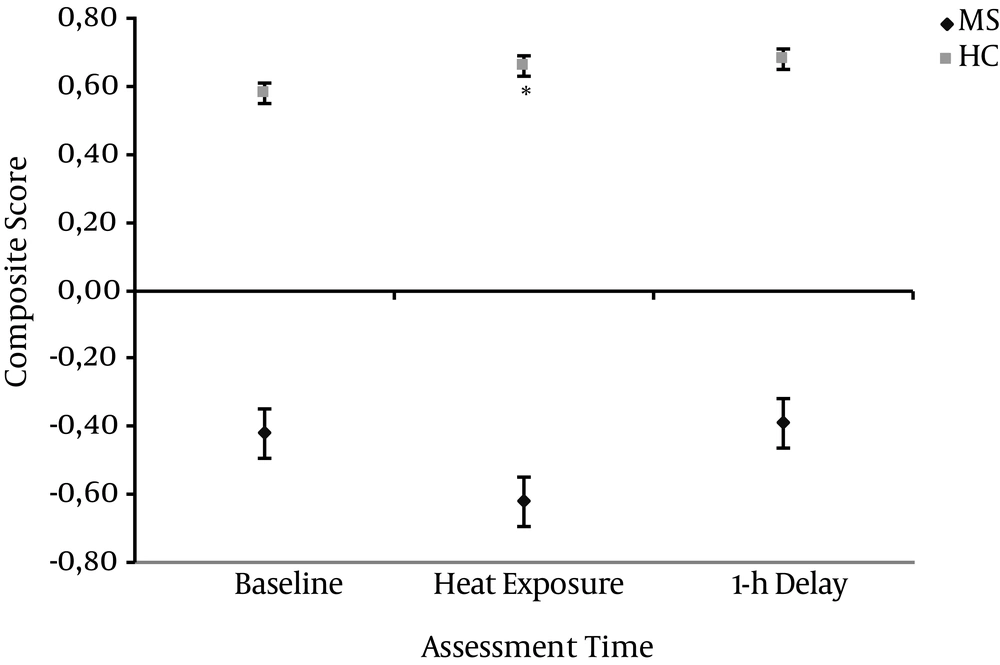
The average MSFC scores of the MS group were -0.48 (SD 0.79) at baseline, -0.99 (SD 1.97) during heat exposure and -0.68 (SD 1.58) after one-hour delay. The average MSFC scores of the HC group were 0.58 (SD 0.42) at baseline, 0.66 (SD 0.43) during heat exposure and 0.68 (SD 0.41) after one-hour delay.
In total, the MS group had significantly lower MSFC scores at baseline when compared to the controls, seen as a significant main effect of group in the repeated measures analysis of variance (ANOVA) (F1, 39 = 5.6, P = 0.01). The comparisons over different assessment points showed significant group-by time interaction in the MSFC scores (F = 1, 58, P = 0). The within-subject pair-wise contrasts showed that the difference from the baseline score to that observed at heat exposure was significantly different between the two groups (F1, 39,P = 0), but not from baseline score to that observed at one-hour delay. The MS patients MSFC score deteriorated in the heat exposure, whereas the HC’s score improved (Figure 1).
The figure illustrates mean MSFC scores of the MS and HC groups at baseline, during heat exposure and after one-hour delay. The error bars represent the standard error of the mean. The MS group’s MSFC score deteriorated due to heat exposure, whereas HC group’s score stayed relatively stable across the three time points (P = 0.01).
5. Discussion
In our previous publications we reported that the core body temperature of MS patients rises more during heat exposure than that of the HCs and that heat temporarily impairs the cognitive and physical performance of MS patients with heat sensitivity (1, 2). In the present study, the data from our previous studies were further analyzed to determine whether the heat-induced decline is observed with the MSFC composite score. In other words, the purpose of the study was to evaluate the compatibility of the MSFC to show the effects of heat in patients with MS.
By re-analyzing the existing data, the present study showed that the MSFC composite score deteriorated in the MS group following heat exposure, whereas the score remained stable or slightly improved in HCs across the three time points. Thus, the MSFC was able to manifest the negative effects of heat on functioning. The MS group had significantly lower MSFC composite score when compared with the HC group already at baseline. This finding is consistent with previous observations on MS patients’ deficient performance in the MSFC (13).
The MSFC is relatively easy and quick to administer, and has been shown to have good intra-and inter-rater reliability (13, 14, 18). The MSFC has also been shown to correlate moderately with the EDSS and with structural neuroanatomical changes observed in the Magnetic Resonance Imaging (MRI) (18, 19). Preliminary studies suggest that the MSFC is better than the EDSS in detecting differences between patient groups and that it is more sensitive to change (18). Furthermore, MSFC-EDSS correlations have been found to be moderately strong, yet more specific analyses have shown that EDSS is strongly correlated mainly to the TWT and only weakly to the 9HPT and the PASAT-3 (18). This confirms the fact that EDSS focuses heavily on the ambulatory function neglecting the changes in hand functions and cognition. While the detrimental effects of heat may be manifested not only in ambulation, a measure covering hand functions and cognition is needed.
The MSFC suffers from a weakness, which had to be taken into account in the present study. The tests of the MSFC, especially the PASAT-3 and 9HPT are vulnerable to practice (18). The MSFC manual recommends three testing sessions before the actual baseline assessment to overcome these effects (16). Rosti-Otajarvi et al. (13) suggested one pre-baseline assessment for the TWT and two for the 9HPT and the PASAT-3 for a Finnish population to compensate for practice. Solari et al. (14) on the other hand suggest that the TWT should be administered once, the 9HPT four times and the PASAT-3 three times before the baseline assessments. In the present study, the subjects practised the TWT and the 9HPT once and the PASAT-3 twice. Still, the HCs slightly improved their performance over the testing sessions.
There were some limitations in our study that have to be taken into account when drawing conclusions. The study groups were relatively small, and the results can be generalized only to patients with relatively mild disability and subjective heat sensitivity. Heat exposure and related assessments were performed right after the baseline assessment, which might have induced overall fatigue in patients with MS. Furthermore, the participants had the opportunity to rest after the second session before the one-hour delay assessments, which might have enhanced the improvement observed in the MSFC at one-hour delay assessment. Further studies are needed to show whether heat also detrimentally affects patients without subjective heat sensitivity, how specific these effects are, and whether MSFC or other simple assessment tools are compatible to show these effects.
In conclusion, the re-analyses of previous data showing detrimental effects of heat on functioning suggest that the MSFC might serve as a simple tool to reveal the negative effects of heat. A brief and relatively easily administrative measure might facilitate the clinical evaluations and offer useful information to diminish the negative effects of heat on patients’ everyday life.