1. Context
In 80 percent of patients with inflammatory pain, including rheumatoid arthritis, life expectancy due to disability reduced from 3 to 18 years. Accordingly, a substantial and increasing body of clinical evidence supports the significant role of probiotics in the inflammatory pain relief and several general mechanisms of action have been proposed (1, 2). Some evidence supports an additional and pivotal role of probiotics as important modulators of immune system responses. In particular, probiotics can affect the inflammatory response by affecting the balance of pro and anti-inflammatory cytokines and mu-opioid receptors (MORs) (3-5). Also, probiotics and some of their secreted products can act as ligands for innate immune system receptors and influencing proinflammatory pathways directly (6, 7).
1.2. Objectives
The purpose of this review was to evaluate the potential anti-inflammatory roles of probiotics during inflammatory disorders and those bacterial compound effects on molecular aspects of inflammatory pain by reviewing different animal and human studies.
2. Evidence Acquisition
In this review, we probed into the effects of probiotics in betterment of inflammatory pain (7). The literature covers a variety of topics such as the definition for probiotics and inflammatory pain, the probiotics selection criteria and the role of probiotics in immune system modulation (6, 8). In this study, we reviewed most of the existing papers on probiotics special focus on inflammatory pain and its related mediators. Our literature review in the electronic databases of MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO yielded 77 articles from 1965 to 2014.
The inclusion criteria of the studies were as follows:
1. Studies on probiotics, the main probiotic bacteria, probiotics selection criteria and suggested mechanisms of their actions.
2. Peruse the effects of probiotics on immune system.
3. Prospective or retrospective studies about inflammatory pain and molecular aspects of inflammatory diseases.
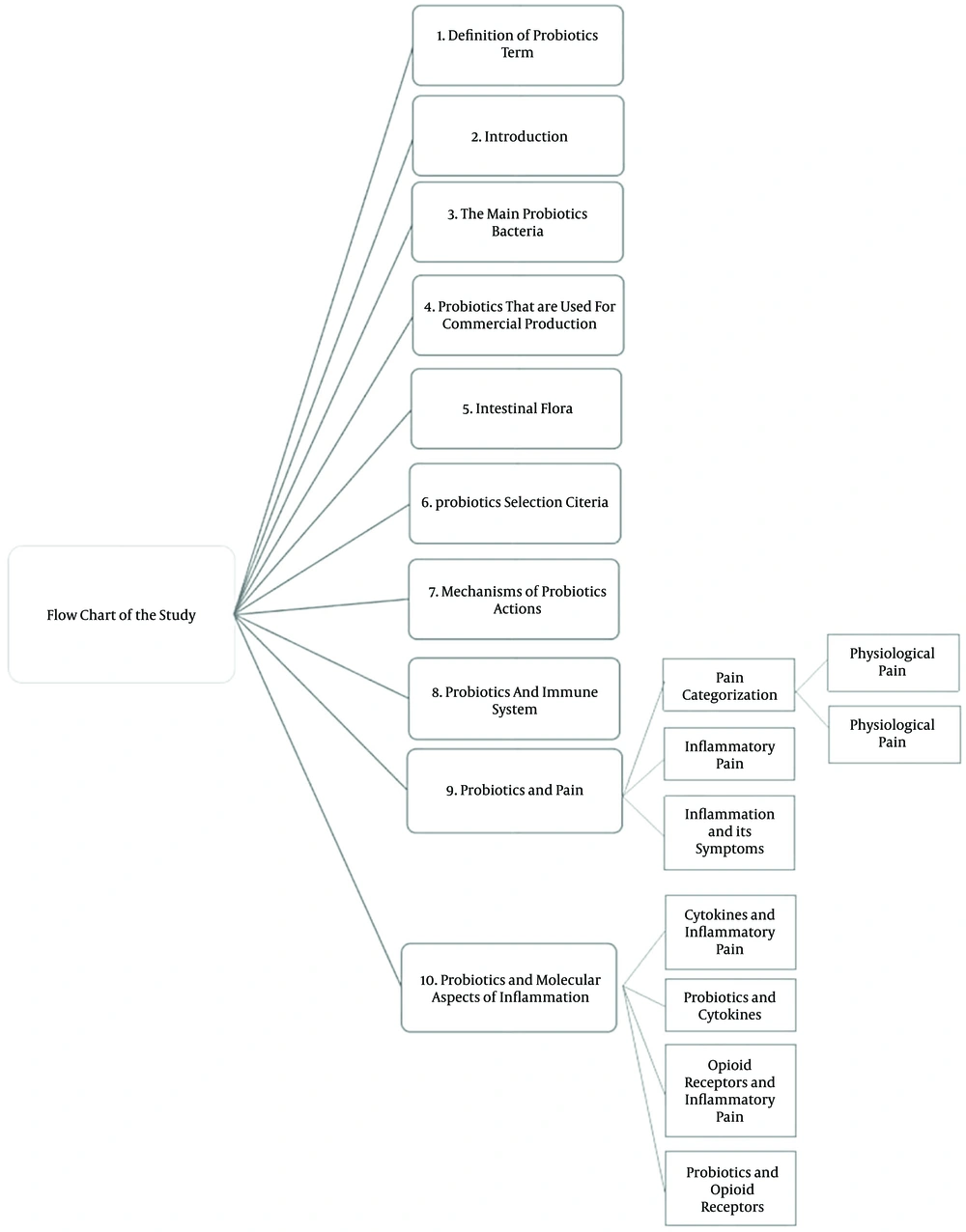
4. Evaluation of the potential role of probiotics on inflammatory pain modulation (Figure 1).
3. Results
A brief summary of the studies is presented below:
3.1. Definition of Term
In 1965, the term “Probiotic” was firstly applied by Stillwell and Lilly to clarify a component secreted by a microorganism and evoked growth of other microorganisms, and then known as the opposite of antibiotics. Following definition is as good as possible sentence could define probiotics: a product containing alive, specified and sufficient enough microorganisms which lead to change the microbial flora by colonization in host, so that they exerted proper action. Studies have shown that even nonliving forms of probiotics also have beneficial effects on health (9, 10).
3.2. Introduction
Most of probiotics belong to a large group of bacteria in the human gut flora and are commensalism and harmless (11). Intestinal flora has a protective role against various diseases. The main effect of probiotics is characterized by stabilizing intestinal flora (12). Main features of probiotics are that contain a large number of living cells, antibiotic resistance, with antimutagenic consequence, without any adverse effect, pathogenicity and toxicity as well (13). They mostly include lactic acid producing bacteria such as Lactobacillus and Bifidobacterium species those would never cause inflammation (9, 10). Lactobacillus bacteria are the most common bacteria applied in probiotic components. Their effects are hardly known. However, they contain many therapeutic and preventive effects such as producing antimicrobial content for pathogen antagonization, intestine pH modulation, bacterial receptors blocking, competing nutrient absorption, lactase producing so alleviate lactose intolerance complications, reducing allergy incidence, demotion risk of cancer and immune system reinforcement. Studies have shown that probiotics, including Lactobacillus bacteria can inhibit the growth of many types of pathological cell (13-15). Lactobacillus bacteria can inhibit tumors growth by stimulating the immune system (for instance increase of lymphocyte cells (T-cells)) (16). Probiotics with direct influence on Peyer’s patch in the intestine can modulate the local immune system and also can affect dendritic cells in the gut wall and lead to the production and proliferation of other cells involved in the immune system such as cytokines (17). Therefore, usage of probiotics can increase the level of immune responses, regulate immune factors, alleviate pain and improve disability in patients with rheumatoid arthritis and it can also improve disorders such as colitis, irritable bowel syndrome and diarrhea. Probiotics are available like dietary supplements and pills and are also present in some dairy products (7, 18).
3.3. The Main Probiotic Bacteria
Probiotics in terms of efficiency can be divided into two categories: bacteria strains which are derived from the intestinal microbiota of healthy humans and nonhuman strains used in the dairy products. The first category, such as L. acidophilus and bifidobacteria are present in all environmental conditions. While the second category, such as Bacillus spp. Enterococcus faecium and Escherichia coli only in special circumstances, for example, only about some of the animals or in the presence of specific bacteria with a particular race, have probiotic properties. Most probiotics belong to the group of lactic acid bacteria (19, 20).
3.4. Probiotic
Probiotics Used for Commercial Production are presented in Box 1 (15, 19-22).
| Species |
|---|
| Lactobacillus species |
| L. acidophilus |
| L. casei |
| L. fermentum |
| L. gasseri |
| L. johnsonii |
| L. delbrueckii ssp. (bulgaricus) |
| L. lacits |
| L. curvatus |
| L. paracasei |
| L. planetarium |
| L. reuteri |
| L. rhamnosus |
| L. salivarius |
| L. cellobiosus |
| L. brevis |
| Bifidobacterium species |
| B. bifidum |
| B. breve |
| B. adolescentis |
| B. lactis |
| B. longum |
| B. thermophilum |
| Streptococcus species |
| S. thermophilus |
| S. cremoris |
| S. salivarius |
| S. diacetylactis |
| S. intermedius |
| Enterococcus species |
| Ent. faecalis |
| Ent. faecium |
| Yeasts and Molds |
| Saccharomyces boulardii |
| Saccharomyces cerevisiae |
3.5. Intestinal Flora
A large number of bacterial species in the intestinal flora are potentially harmful because they produce toxins, and have the ability to activate carcinogens leading to mucosal invasion and inflammatory responses (16, 23). Also, 10 - 20 species of bacteria in the intestinal flora are dominant, which are as follows:
Peptococcus, Eubacterium, Clostridium, Lactobacillus, Bacteroides, Veillonella, Bifidobacterium, Fusobacterium, Escherichia and Peptostreptococcus.
Bacteria that have beneficial effects on human health are Bifidobacterium and Lactobacillus (22). The main functions of the intestinal flora can refer to metabolic activity that can lead to conserve energy and nutrients and also have important trophic effects on intestinal epithelial cells, immune functions and protect the host against invading alien microbes. In infectious diseases and inflammatory conditions, intestinal ecology balance change in a way of increment the number of pathogenic bacteria and immune responses by this group of bacteria that inhabit in the gut can induce. Among these diseases can note rheumatoid arthritis, and allergic diseases (24, 25).
3.6. Probiotics Selection Criteria
1. Be safe and nonpathogenic and belongs to generally recognized as safe (GRAS),
2. Resistance and survival potential in the manufacturing technology process,
3. Stay live and active in the gastrointestinal tract, which means having stomach acid and bile acid resistance,
4. The ability to antagonize the pathogens by producing antibacterial compounds or competition eliminating via reducing colon pH,
5. The ability to bind to intestinal epithelial cells,
6. The ability to stabilize the intestinal bacterial flora,
7. Resistance to bacteriophage,
8. Have genetic stability,
9. Do not transfer antibiotic resistance genes,
10. Able to induce an immune response without causing inflammation,
11. Antimicrobial activity against pathogenic bacteria such as Helicobacter pylori, Salmonella, Listeria monocytogenes, Clostridium difficile, Bacillus and Enterococcus species (13, 19, 21).
3.7. The Main Suggested Mechanisms of Probiotics Actions in the Body
1. Probiotics produce inhibiting compounds and antimicrobial activities. Also, probiotics produce different antibacterial compounds such as H2O2, propionate, butyrate, acetate, diacetyl, organic acids, acetaldehyde, lacto peroxidase, lactones, CO2 and high molecular weight compounds such as bacteriocins and defensins, which have inhibitory effects on Gram-positive and Gram-negative bacteria. This combinations not only reduce the number of living cells of the pathogens, but also may affect the metabolism of bacteria or toxins produced by them (2, 26).
2. Competition for bacterial binding sites on the surface of intestinal epithelium. Today, it is accepted that many enteric pathogens to situate in the gut and cause diseases should bind to the intestinal wall. Some strains of probiotics have the ability to connect to epithelial cells and then bacterial binding sites are blocked (1, 27-29).
3. Intensification of barrier function: probiotic bacteria can enhance mucus production and increment the barrier function to exert their effects (2).
4. Competition for food: probiotics may use some foods that are consumed by pathogenic bacteria (29).
5. Eliminate toxin receptors: it is a hypothetical mechanism and not yet proven and more studies are needed (30).
6. Production of nutrients: probiotics through the production of vital nutrients such as short-chain fatty acids, vitamins, amino acids like arginine, glutamine and cysteine, polyamines, growth factors and antioxidants play an important role in maintaining health (31, 32).
7. Metabolic actions: probiotics may reduce mutagenic and toxigenic activity in the intestine. Also, they can decline the serum cholesterol by affecting the bile salt conjugation and secretion. Probiotics can improve lactose intolerance through lactose hydrolysis (15).
8. Reinforcement of the immune system: probiotic bacteria have effects on epithelial cells, dendritic cells, monocytes/macrophages, B-lymphocyte, NK cells and T-cells redistribution and by adjusting these factors can have an important role in modulating the immune system function (1, 2). Because of the importance of this part we devoted a section to probiotics and immune system.
3.8. Probiotics and Immune System
The immune-boosting effects of probiotics are able to increase levels of anti-inflammatory cytokines and immunoglobulins, enhancement of mononuclear cell proliferation, activation of macrophages, the intense activity of natural killer (NK) cells, autoimmune modulation and immune stimulation against pathogenic bacteria and protozoa (33-36). Studies have shown that all the probiotic bacteria lead to increase of immune cell proliferation and diminish the production of proinflammatory cytokines such as TNF-α and IL-6 (37, 38). Also, probiotics suppress lymphocyte proliferation and proinflammatory cytokines production by T cells. Also, probiotics can down regulate T-helper1 responses. The positive effects of probiotics on the immune system are without harmful inflammatory responses (2, 22). When probiotics are used together (for example combination of Lactobacillus and Bifidobacterium) and can act synergistically, they are able to boost their effects on the immune system. Effectiveness of probiotics against bacterial infections and immunologic disorders such as asthma in adults, cancer, diabetes and rheumatoid arthritis in human has been demonstrated. Probiotics produce IgA and anti-inflammatory cytokines and by enhancing phagocytosis of pathogens, can stimulate and strengthen the nonspecific immune system (6, 39-41). Probiotics can modulate phagocytosis in healthy and allergic people in different ways. In healthy individuals, probiotics are able to stimulate the immune system and reduce the inflammatory responses in allergic individuals (34, 41, 42).
3.9. Probiotics and Pain
a) Pain Categorization:
International association for the study of pain (IASP) defined pain in this way: pain is an unpleasant emotional feeling resulting from actual or potential tissue damage that can be associated with cognitive processes and emotional responses (43).
According to the function in the body, pain is divided into two types:
1) Physiological pain; one of the mechanisms of body protection that is created by pathological lesions (damaged tissue damage, neuropathic, inflammatory, etc.) and caused by stimulation of sensory peripheral receptors of pain and help the person to aware of the harmful stimulus in order to remove painful stimulus with protective reflexes that ultimately can maintain homeostasis (44, 45).
2) Pathological pain: is a type of pain that results from direct damage into the peripheral or central nerves, which in this case increases the activity of neurons in the pain pathways and by removing harmful stimuli, pain does not go away (45).
b) Inflammatory Pain:
Inflammatory pain caused by direct stimulation of nociceptors by release of inflammatory mediators such as bradykinin, histamine, cytokines and chemokines (46, 47). Increased levels of proinflammatory cytokines and activation of intracellular signaling pathways induced by these factors are the main cause of inflammatory pain development. Scientists also believed that inhibiting the action of the proinflammatory cytokines may be an important strategy in the treatment of inflammatory pain (8, 48).
c) Inflammation and Its Symptoms:
Inflammation is a local response through tissue damages and as a first physiological defense mechanism of the body helps to protect the body against damaging stimuli along with the release of a series of different mediators such as bradykinin, histamine, chemokines, signaling pathways and cytokines particularly interleukin-1(IL-1), interleukin-6 (IL-6) in blood cells and damaged tissue (22, 47-49). Chronic inflammation can induce changes in the affected area. The persistence of inflammation causes some symptoms like redness, swelling (edema), reduced motor activity in the affected area, pain and rise of temperature that treatment of each of these symptoms is effective in healing inflammation (49-51).
3.10. Probiotics and Molecular Aspects of Inflammatory Pain
a) Relation Between Cytokines and Inflammatory Pain:
During an inflammatory pain, proinflammatory cytokines (e.g. IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α)) were elevated and anti-inflammatory cytokines (e.g., IL-10 and transforming growth factor beta (TGF-β)) decremented (33, 50, 52, 53) (Figures 2 and 3). Inflammatory cytokines have beneficial effects on the host, but if they were over-expressed, they can induce pathological conditions (48, 54-56). Inflammatory cytokines and chemokines can change the animal response to the painful stimulus and cause some changes such as hyperalgesia, allodynia and edema (56). Continuation of the release of proinflammatory cytokines during inflammatory diseases is associated with glial cell activation and transmission of inflammatory intermediate factors through the blood-brain barrier can induce inflammatory pain (8, 57). Cytokines play an important role in initiating the inflammatory cascade and are able to induce the production and secretion of signaling molecules, which are able to produce inflammatory mediators. Several groups of mitogen-activated protein kinase (MAPK) in the signal transmission pathway of cytokines have been identified (50, 58). Previous studies have shown that intracellular signaling pathway activity during inflammation induces elevation of production and secretion of proinflammatory cytokines, which can cause inflammatory symptoms (50, 59). Studies have shown that the usage of monoclonal antibody for inhibition of proinflammatory cytokines and intracellular signaling pathways can decrement the symptoms of inflammatory disease and it can be an important strategy in the treatment of these diseases (60-62). Interleukin-10 as an anti-inflammatory cytokine can restrain the synthesis of proinflammatory cytokines such as TNF-α, IL-1, Granulocyte-macrophage colony-stimulating factor (GM-CSF) and HLA class ІІ. Increment of anti-inflammatory cytokines can be effective in alleviating the symptoms of inflammation and pain (63, 64).
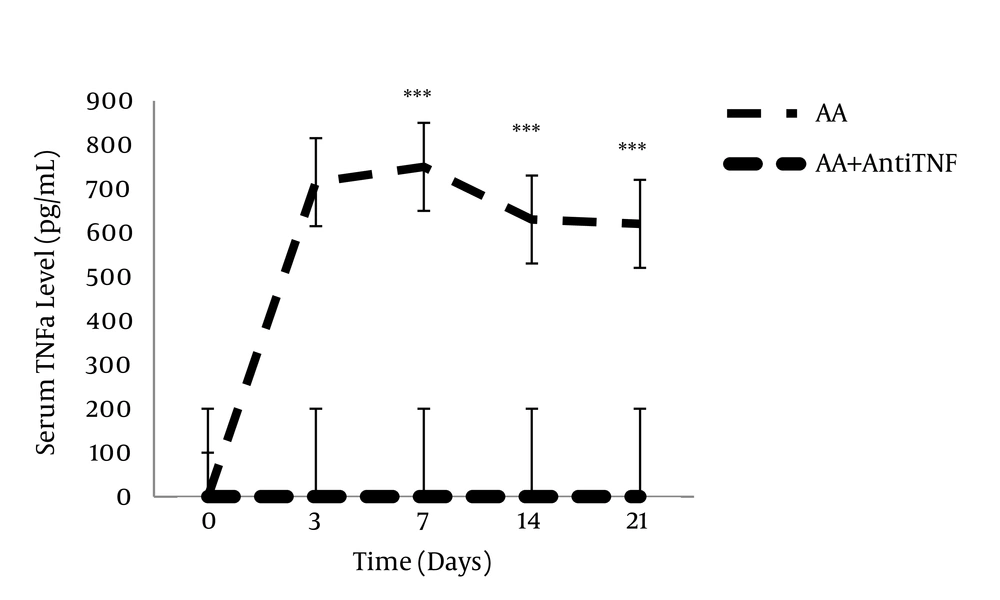
This illustration shows the enzyme-linked immunosorbent essay (ELISA) analysis of the serum TNFα level during different stages of complete Freund’s adjuvant (CFA)-induced inflammation. A significant decrease in the serum TNFα levels were observed in adjuvant-induced arthritis (A.A) + anti- TNFα group. Data are presented as mean ± SEM (n = 6/group). ***P < 0.0001: for comparing the variation of the serum TNFα level between different days of study with day zero (Akhtari et al. 2012) (65).
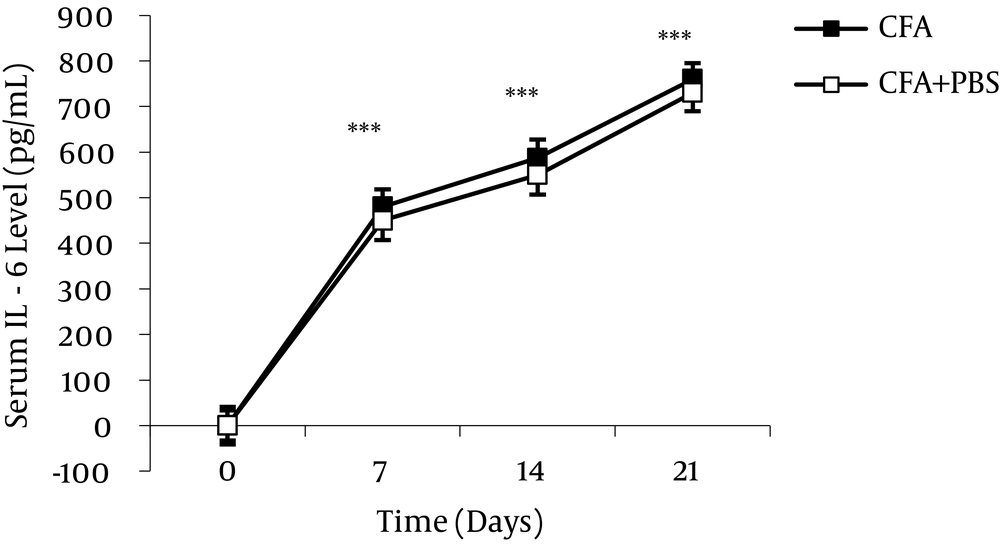
The serum IL-6 level significantly increased during different stages of AA. Anti-IL-6 antibody treatment during AA caused significant reduction in the serum IL-6 level. Data are presented as mean ± SEM (n = 6/group). ***P value < 0.001 indicates serum IL-6 increase during different stages of AA compared to day 0 (Alani et al. 2011) (66).
b) Effects of Probiotics on Cytokines:
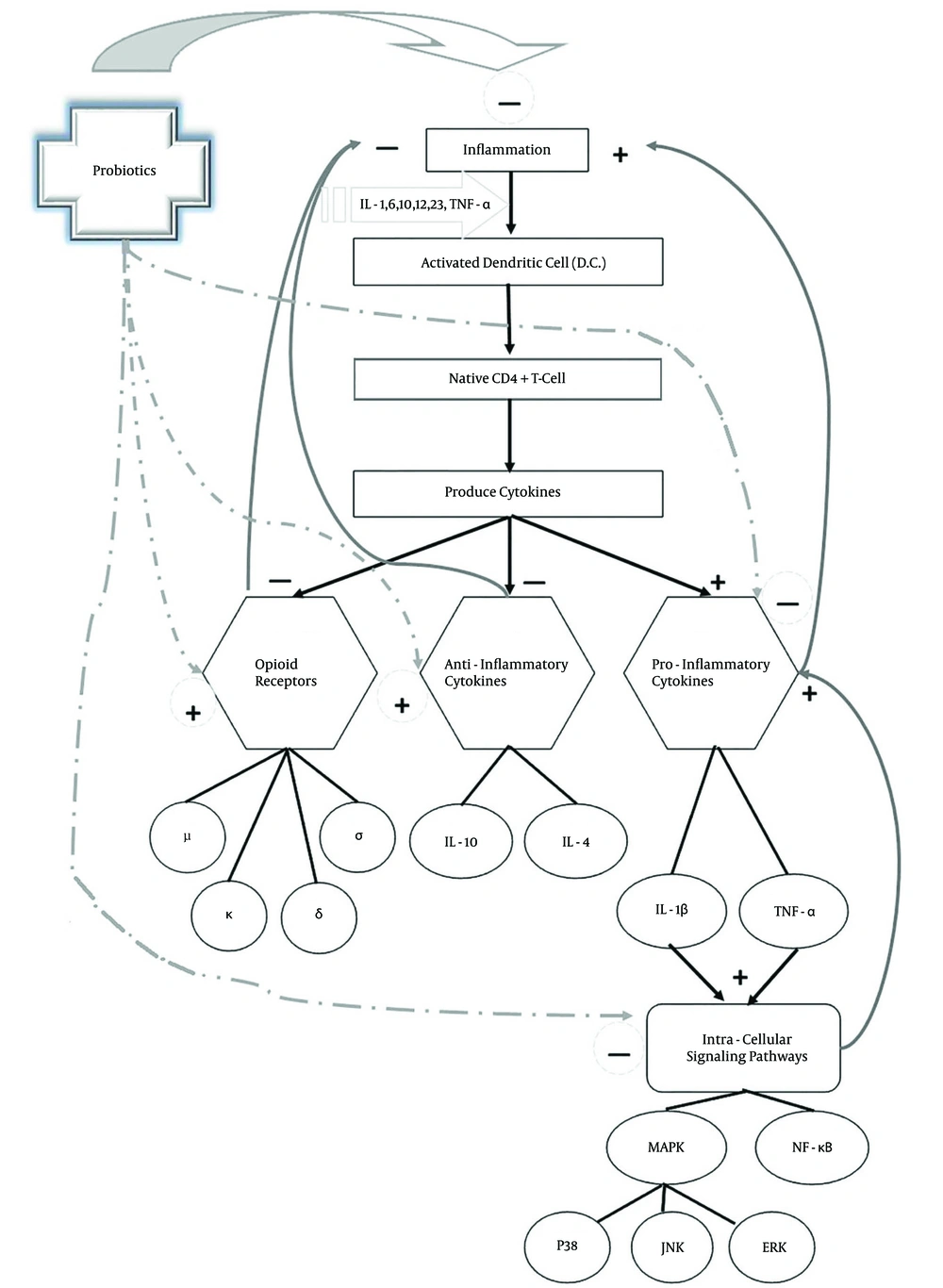
Many studies have shown that probiotics are able to adjust the balance of proinflammatory and anti-inflammatory cytokines and increase immunoglobulins levels like IgA. Studies have shown that probiotic drugs are capable to modulate the immune system, down regulate the inflammatory factors of immune system, decrement proliferation of T-Cells, reduction of proinflammatory cytokines (including IL-1β, IL-2, IL-6, IL-12, IL-17, IFN-γ, tumor necrosis factor alpha), decrease of NF-κB and other intracellular signaling pathways mediators, decline of C-reactive protein and increase regulatory and anti-inflammatory cytokines (IL-10, TGF-β) (5, 6, 18, 33). Then, it seems that probiotics are effective to elevate the level of anti-inflammatory cytokines and diminish the level of proinflammatory cytokines and thus capable to modulate the immune system. So, they can have an effective role in the betterment of inflammatory pain symptoms (6-8, 67) (Figure 4).
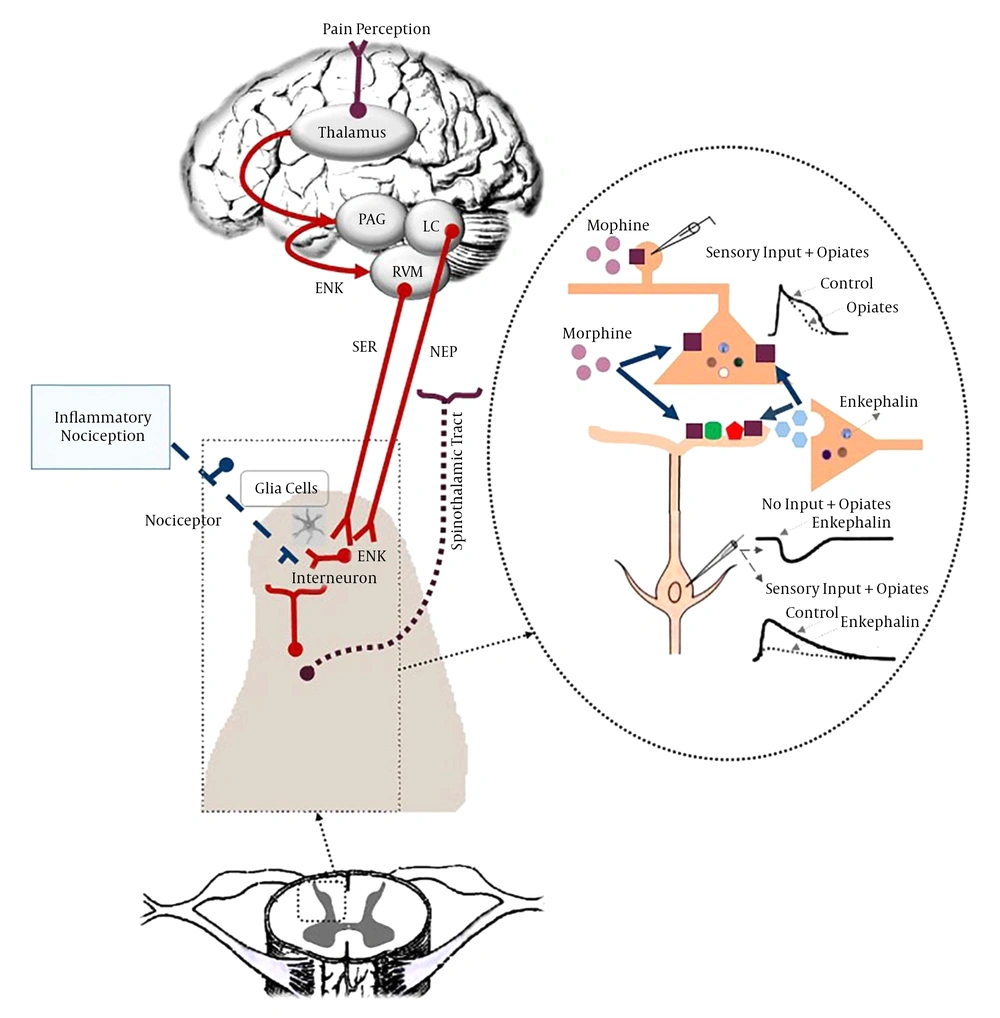
c) Role of Opioid Receptors in Inflammatory Pain Modulation:
Opioid receptors in the peripheral terminals of sensory neurons are involved in pain modulating system and play a significant role in the moderation of hyperalgesia in inflamed tissues. There is a close correlation between the expression of cytokines and opioid receptors in the process of inflammation (59) (Figure 5). Cytokines may modulate the de novo synthesis of opioid receptors in effector immune cells and neuronal cells. Opioid agonists reduce pain in inflamed tissue (59, 68). Studies have shown that long-term administration of anti-IL-6 effectively diminishes the expression of opioid receptors during chronic phase of inflammation. Evidence has shown that MAPKs play a consequential role in adjustment of opioid receptors endocytosis (59). Some anti-inflammatory cytokines cause analgesia through activating the endogenous opioid system in inflammation (59, 69).
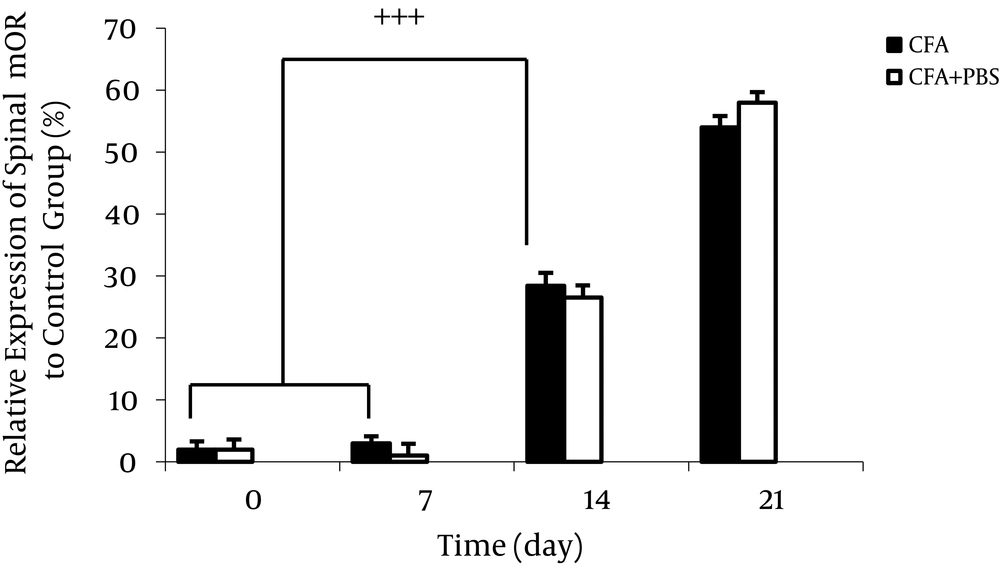
The complete Freund’s adjuvant injection caused significant increase in spinal mu-opioid receptors (MOR) expression time dependently. Data are presented as mean ± SEM (n = 6/group). +++P < 0.001 indicates comparison of ratio of spinal MOR protein band intensity between different days of AA (Alani et al. 2011) (66).
Between opioid receptors, µ-opioid receptors (MOR) are most commonly mediators associated with analgesic therapy in chronic pain. Previous studies have shown that inflammation affects the expression of MOR. In the process of inflammation, opioid receptors are increased which is related to its antinociceptive features and followed by hyperalgesia reduction (64). Some studies have stated that in acute phase of inflammation, the number of binding sites of MORs in the hypothalamus and spinal cord boost. In general, fluctuation of MORs expression during inflammatory pain can affect hyperalgesia. Mu-opioid receptors play an influential role in pain modulation (68, 70, 71) (Figure 6). Philippe et al. have shown that MOR expression was up-regulated in mucosal samples from human patients with inflammatory bowel disease (IBD) compared with control samples and these data implicated the notable role of the MOR signaling pathway in gut homeostasis regulation (70). The MOR signaling pathway via down regulation of cytokines production and modulation of T-cell functions can improve intestinal inflammation, but the mechanisms of this effect have not been well recognized yet (70, 71). Mu-opioid receptors can decrease intracellularcyclic adenosine monophosphate (cAMP) through inhibiting adenylate cyclase. Also, they reduce neuronal excitability (via hyperpolarization resulting from elevated membrane potassium channels permeability) and neurotransmitter release (via inhibition of voltage-gated calcium channels) (72).
d) Probiotics and Opioid Receptors:
Probiotics can induce the expression of μ and κ-receptors in peripheral nerve fibers and increase the number of peripheral nerve terminals in chronically inflamed tissue (72-74) and also can activate and enhance expression of opioid receptors on epithelial cells which locally control the transmission of nociceptive information to the intestinal nervous system (75).
Probiotics via influencing and augmenting the expression of cannabinoid and opioid receptors can decline the hypersensitivity and mediate analgesia thereupon alleviate pain (3, 4, 76). Furthermore, the peripheral mu-specific agonist can alleviate inflammation (71). However, little is known about the regulation of these opioid receptors, their mechanism of action, and whether they demonstrate a class of molecules, strategically placed in specified gut epithelial cell lineages which sense the changes in biochemical environment of the gut and transduce information toward key metabolic activities of the microbiota, like polysaccharide fermentation, in ways that impact host physiology, including energy balance (77).
4. Conclusions
According to the role of intestinal flora in pathogenesis of autoimmune and inflammatory diseases, some studies indicated the role of probiotics in modulation of microbial flora and betterment of inflammatory symptoms during different systemic disorders. Some of these effects carried out via reduction of proinflammatory cytokines and elevation of anti-inflammatory cytokines. Also, due to the impact of probiotics on the expression of opioid receptors and the role of these receptors in inflammatory pain alleviation, it seems that probiotics exert some parts of their analgesic effects in this way.
Accordingly, it appears that regular and controlled use of these bacterial compounds can be a major step forward in the modulation and treatment of inflammatory symptoms in inflammatory diseases but more clinical studies are needed.