1. Introduction
Myalgic encephalomyelitis (ME) is a debilitating illness, and more research is needed to develop and evaluate interventions for those affected. Many patients have been stigmatized by this illness (1). For example, Twemlow et al. (2) found that individuals with ME reported that they were made worse by their health care workers 66% more often than general medical patients. It is unfortunate that there have been few funded trials of pharmacological or non-pharmacological interventions for patients with ME. Evaluations of cognitive behavioral interventions have been mixed. Price et al. (3) reviewed 15 studies of CBT with a total of 1,043 CFS participants. At treatment’s end, 40% of people in the CBT group showed clinical improvement in contrast to only 26% in usual care, but changes were not maintained at a one to seven-month follow-up when including patients who had dropped out.
Operant oriented interventions may provide good ways to help patients, as they stress the importance of involving the target populations for input concerning such aspects as problem identification, information on the problem, intervention design, and intervention acceptability (4, 5). As long as the issues are well defined, this approach can be a tool whereby clinicians and patients jointly plan and implement interventions as true partners. When evaluating their interventions, behaviorists collect time-series data that are objective and quantifiable using a variety of experimental designs, including reversal (ABAB) designs; multiple-baseline designs across time, individuals, settings, or situations; changing-criterion designs; and multiple treatment designs (6). In the basic time-series model, data are collected until a stable baseline rate for some dimension of behavior has been established. Intervention is then introduced while data continue to be collected. If a change that is large, relatively immediate, and socially substantive is apparent, a stable change as a result of intervention is regarded as present. The standard form of analysis in behavioral designs is visual, accepting only clearly evident and reliable changes as depicted graphically (7).
The current study involved two patients with ME, and each collected data over time during a baseline period, and then initiated interventions to determine the outcomes. We hypothesized that patients would be able to both collect this information on a regular basis, and that the collected data would provide useful feedback as to the efficacy of the interventions.
2. Case Presentation
Both participants were part of the HealClick (now called MyPatientMatch) online patient community, where they had an opportunity to post the interventions they were using as well as their charting of symptoms over time. Because the data of the participants was provided voluntarily to the online patient community and anonymized before we were given access to it, we were not required to obtain IRB approval from our university.
The first participant was a 54 year old female and had previously been a speech therapist. She had been diagnosed by her physician with ME, and had not been able to work over the past 9 years. She had been eating a high-sulfur diet for years, and was a vegan. Protein-rich foods, such as fish, poultry, meats, nuts, as well as chocolate are dietary sources of sulfur. Efforts to treat her ME included hormone therapy, naltrexone, COQ10 (50 mg), and hydroxyform B12. As none of these treatments worked, her doctor suggested going on a low-sulfur diet in June of 2014. Low-sulfur foods include: tomatoes, whole wheat, oats, peaches, cantaloupe, coconut, corn, celery, almond milk, bananas, salad, berries, green pepper, and cucumbers. Her doctor felt that some individuals have major inflammatory reactions when they consume high-sulfur diets, and those with sulfur intolerance typically have a combination of genetic polymorphisms and small intestinal bacterial overgrowth. Two of her primary symptoms included brain fog and lack of well-being. She also had high levels of post-exertional malaise.
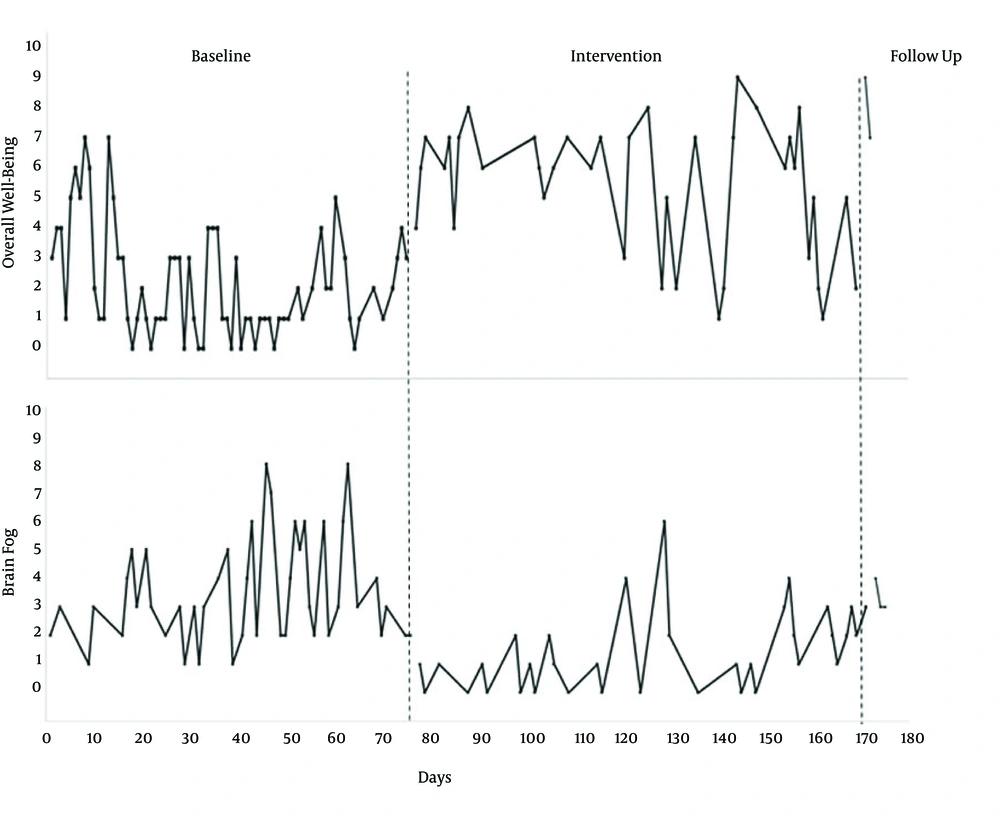
Figure 1 shows her baseline data for overall well-being (top figure) and brain fog (bottom figure). These two dimensions were rated on a 10-pt scale, with higher numbers indicating better well-being and higher brain fog. After day 78, she began the intervention and well-being increased and her brain fog decreased. Data was collected until day 170. In addition, at a year follow-up, her brain fog continued to be decreased and her well-being tended to fluctuate but was better than baseline data. Even though there was improvement on these domains, she still had extreme fatigue and post-exertional malaise.
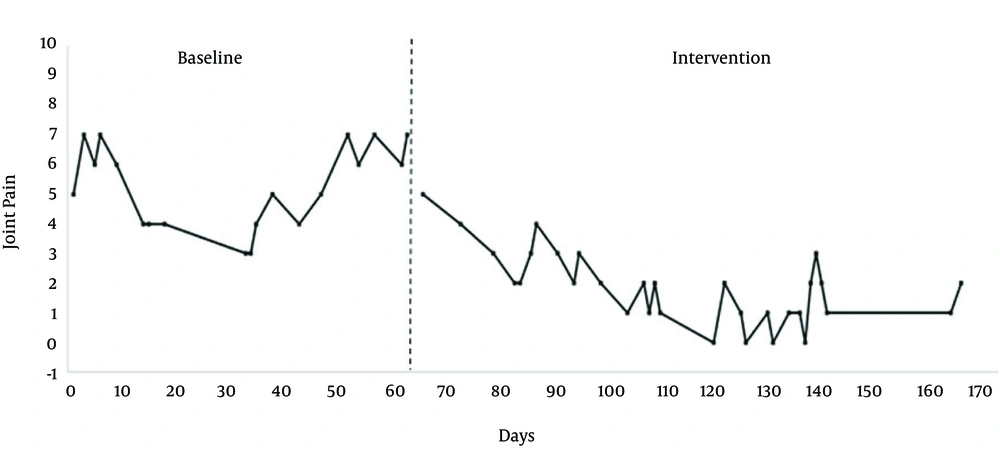
The second patient was a 45 year old female, who had been diagnosed with ME 4 years ago. As indicated on Figure 2, she selected joint pain as the symptom to chart over time. This symptom was scored on a 10-point scale, with higher scores indicating more pain. Although this was not her most important symptom, she did feel pain on an hourly basis and wanted to find ways to alleviate it. After a baseline period, which lasted for 63 days, she began a mold avoidance intervention. Exposure to mycotoxin producing mold is a significant health risk and Brewer et al. (8) found urine samples from 93% of patients with CFS were positive for at least one mycotoxin. After beginning this mold avoidance intervention, the patient noticed that some of that pain was reduced. She also reported sleeping better and being more cheerful, and she did not have to expend so much of her time and energy trying to deal with the ongoing pain. Her intervention involved moving out of her apartment building, which had mold within it, and throwing away clothing that was saturated with mold, but she did keep metal, glass, and ceramic items. The patient moved to a place in the country. Data was collected until day 168, but follow-up data was not available.
3. Discussion
The current two case studies provide illustrations of how patients can be empowered to evaluate the effects of their interventions by collecting and analyzing data over time. Using such operant methods, patients can learn to rate behaviors and assess how well interventions work over time. In some cases, interventions might be effective for a period of time and then lose their effectiveness. With other situations, the interventions might not be effective at all. This type of data can be used to inform patients of whether they should continue using particular interventions for specific conditions or symptoms, as was illustrated with the two case studies.
Other work with ME has shown that non-pharmacologic intervention endeavor to help patients self-monitor and self-regulate energy expenditures and learn to pace activities and stay within their energy envelope (9). Interventions that challenge basic patient illness beliefs may solidify already negative attitudes of medical personnel toward people with ME. The approach we have illustrated in the two case studies represent alternative approaches for helping patients with ME. This approach involves helping patients better monitor symptoms as they attempt lifestyle changes that can be evaluated.
Many patients with ME try multiple interventions and due to this, it is often difficult to know which ones are effective and which ones are not. The operant methods illustrated in this article provide one way of dealing with this problem. In addition, this method does not require expensive clinical trials, but each patient can learn to be an investigator for their own particular set of symptoms and possible interventions. These data can then be used cooperatively to monitor changes. Certainly, these are only case studies, and as such, there are limitations to the data presented herein, particularly as reversal or multiple baseline designs were not employed. However, these operant methods do provide both patients and clinicians with powerful and flexible methods for evaluating changes among patient symptoms over time.