1. Introduction
Nevoid basal cell carcinoma syndrome (BCNS) or Gorlin-Goltz syndrome is an autosomal dominant inherited disorder with high penetrance and variable expressivity (1). This syndrome was first reported by Jarish in 1894, who described a patient with multiple basal cell carcinomas, scoliosis and learning disability (2). Patients with BCNS have a germ-line mutation in the PTCH gene, a tumor suppressor gene and the human homologue of the Drosophila patched gene (3). The prevalence of BCNS has been estimated to be about 1 in 19000 to 1 in 256000 with a male-to-female ratio of 1:1 (4-6). In 1960, Robert Gorlin and Robert Goltz determined the condition as a syndrome comprising a triad of multiple basal cell nevus, jaw keratocysts, and skeletal anomalies (7). Other terms of this disease include basal cell nevus syndrome, nevoid basal cell carcinoma syndrome (NBCCS), Herman-Grosfeld-Spaas-Valk syndrome, multiple basal cell carcinoma syndrome and hereditary multiple cutaneomandibular polyoncosis (1).
Multiple organ systems may be affected in Gorlin-Goltz syndrome including abnormalities of the skin, the skeletal system, genitourinary system, and central nervous system. The five principal clinical features of BCNS comprise of multiple basal cell carcinomas (BCCs), which often develops at young age, odontogenic keratocystic tumors (OKCs) that appear in the first, second and third decades of life, congenital skeletal anomalies, intracranial calcification, and multiple Palmar and/or plantar pits (7-9). The patient often has a characteristic facies with frontal and temporoparietal bossing, which results in an increased cranial circumference (> 60 cm in adults). The eyes may appear widely separated and many patients have true mild ocular hypertelorism. Mild mandibular prognathism is also commonly present (6).
The diagnosis of BCNS is based on clinical, radiological findings and familial history of the patient. Basal Cell Carcinoma Syndrome is diagnosed with two major criteria or one major plus two minor criteria. The diagnostic criteria of this syndrome are summarized in Box 1 (10). Genetic counselling must be considered. Furthermore, ultrasound scans during pregnancy can help detect developmental malformations. The patients are usually referred to the dermatologist with complaints of multiple skin lesions. However, depending on the amount and severity of symptoms, management of the disease may require a wide range of specialists such as dentists, cardiologists, oncologists and orthopaedic surgeons (11).
| Criteria |
|---|
| Major criteria |
| 1. More than two basal cell carcinomas (BCC) or one BCC in patients < 20 years |
| 2. Histologically-proven OKCs of the jaw |
| 3. Three or more cutaneous palmar or plantar pits |
| 4. Bifid, fused or markedly splayed ribs |
| 5. First degree relative with BCNS |
| Minor criteria |
| 1. Proven macrocephaly, after adjustment for height |
| 2. Congenital malformations: cleft lip or palate, frontal bossing, ‘coarse face’, moderate or severe hypertelorism |
| 3. Other skeletal abnormalities: Sprengel deformity, marked pectus deformity, marked syndactyly of the digits |
| 4. Radiological abnormalities: Bridging of the sella turcica, vertebral anomalies such as hemivertebrae, fusion or elongation of the vertebral bodies, modeling defects of the hands and feet, or flame shaped lucencies or the hands or feet |
| 5. Ovarian fibroma |
| 6. Medulloblastoma |
In this paper, we report on a case of BCNS in a 26-year-old male patient that was diagnosed by clinical and morphological criteria.
2. Case Report
A 26-year-old male was referred to the orthodontic department of dental school of Isfahan University of Medical Sciences for dental occlusion abnormalities evaluation and treatment on the 16th of April 2013. He was complaining of a painless swelling in the left posterior region of the mandibular bone. According to the patient, the swelling has appeared five months ago with slow growth (Figure 1).
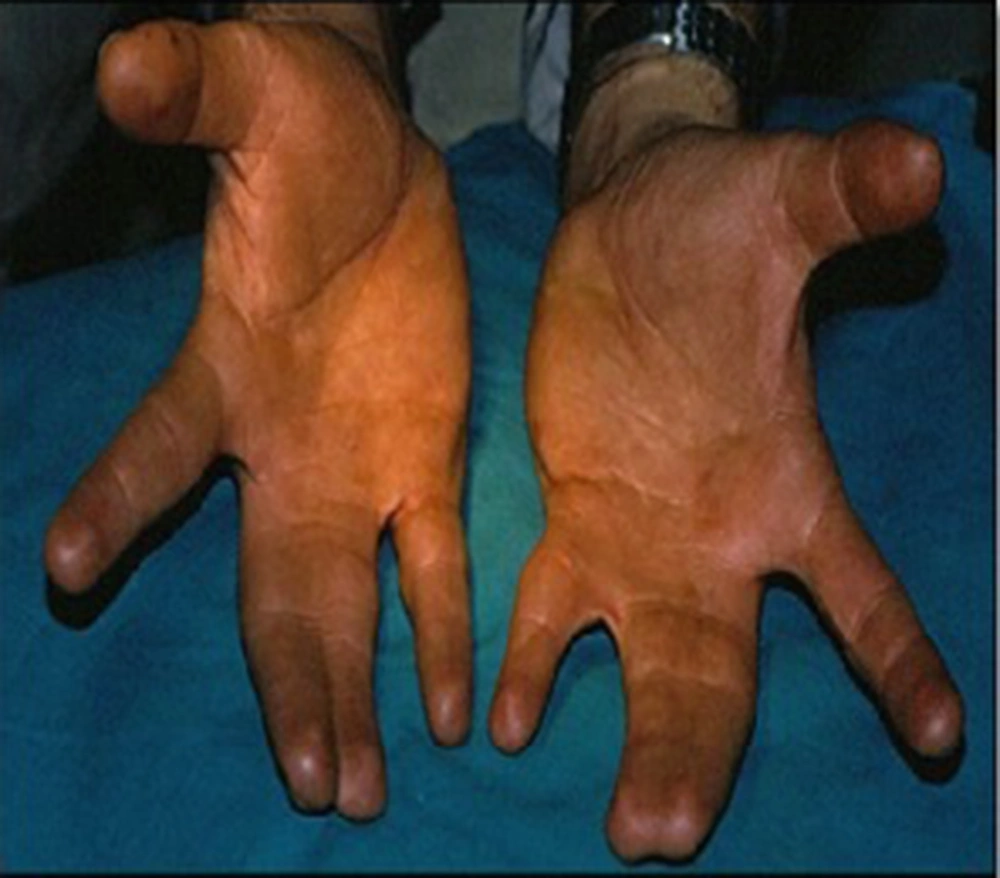
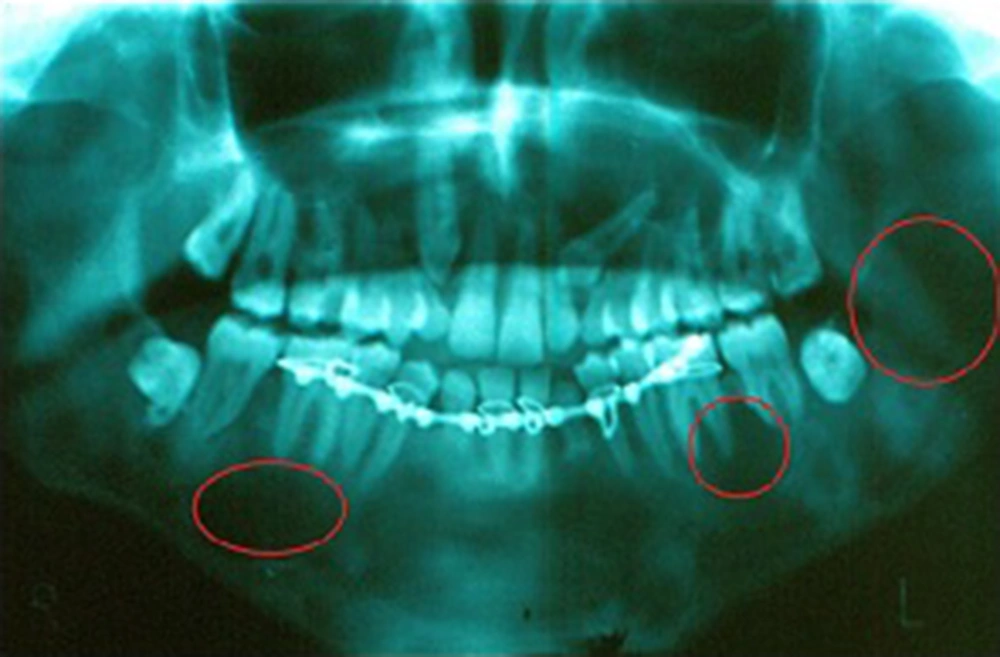
Clinical examination revealed finger syndactyly in both hands (Figure 2). The panoramic X-ray displayed bilateral, multiple unilocular and multilocular well-defined radiolucency in the posterior region of the mandibular bone and multiple impacted teeth (Figure 3).
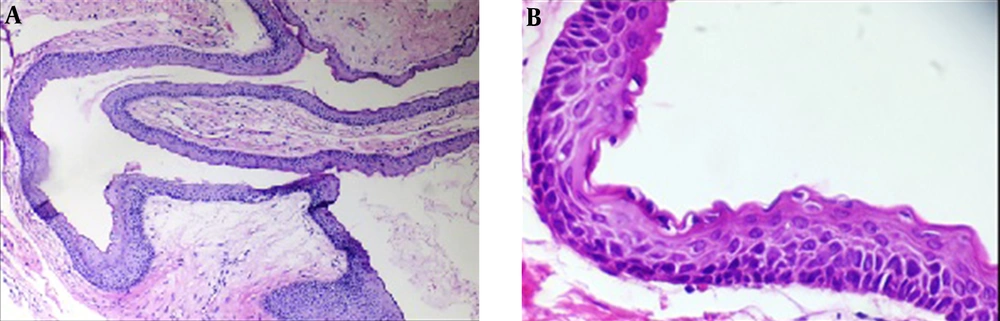
After incisional biopsy, the histopathologic evaluation of the jaw lesions evinced all being odontogenic keratocyst (OKC). The histology of OKC was characterized by parakeratinized stratified squamous epithelium with a wavy or corrugated surface. Epithelium was five to eight layers thick without rete ridges. Basal layer was columnar or cuboidal cells with hyperchromatic nuclei and palisaded appearance. Epithelial islands and daughter cysts were not seen in the fibrous connective tissue (Figure 4).
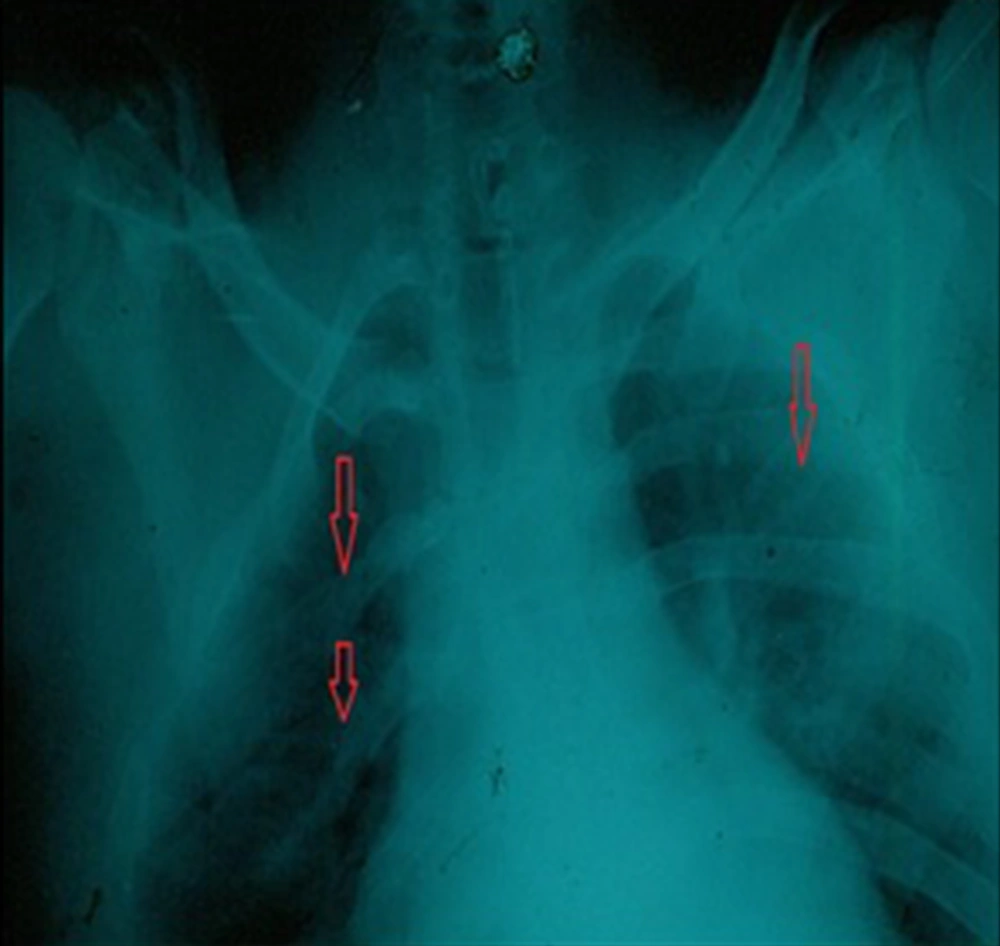
As the patient was suspected of Nevoid basal cell carcinoma syndrome, a chest X-ray was done and showed a bilateral bifid rib (Figure 5). The presence of two major criteria (OKC of the jaw and bifid rib) and one minor criteria (syndactylism) confirmed BCNS. The patient underwent treatment by enucleation and curettage of the jaw cysts and extraction of the impacted teeth.
Two years and six months after last surgical treatment, no recurrence of cysts in panoramic X-ray controls were detected.
3. Discussion
Nevoid basal cell carcinoma syndrome or Gorlin-Goltz syndrome is diagnosed based on clinical examination and radiographic evaluation (7). The physical examination must contain skin and oral evaluation, cephalic diameter, craniofacial morphology. Chest X-ray and panoramic radiography are required. Brain magnetic resonance imaging (MRI) for the detection of meningiomas or medulloblastoma maybe needed. Furthermore, ovarian ultrasound and echocardiography for assessment of ovarian cysts or cardiac fibroma was suggested (7, 12). The diagnosis of Gorlin-Goltz syndrome can be confirmed by the detection of a mutation in the PTCH gene (8). However, there is no relationship between the genotype and phenotype of this syndrome that suggests very complex variability of the phenotype (13).
Odontogenic keratocysts appear in 75% of the syndromic patients and are normally the first symptoms (14). The odontogenic keratocysts in BCNS usually involve unilocular or multilocular radiolucencies of the posterior body, angle or ramus of the mandible. The lesions are often bilateral, although can be unilateral (15). Syndromic OKC has a higher recurrence after treatment and more aggressive behavior than sporadic lesions (16). Because of the intrinsic growth potential of epithelium lining of OKC, they have been termed odontogenic keratocyst tumors (3). In young patients, the cysts may be associated with unerupted teeth and occasionally may cause displacement of teeth or root resorption (15).
In our case, bilateral radiolucencies in the posterior body of the mandible were seen. The jaw lesions were present with impacted and erupted teeth. However, no evidence of root resorption and displacement of teeth were observed. His jaw cysts had typically histologic feature of odontogenic keratocyst.
In syndromic OKC, the connective tissue wall contains small islands of epithelial and satellite or daughter cysts. These lesions had a strong tendency for recurrence after treatment, maybe due to returns of the thin lining and satellite cysts in the capsule of the cysts (15). Therefore, there is a need for long-term follow-up of syndromic patients. More radical surgical resection maybe needed for large OKC particularly in syndromic patients but the extensive nature of the surgery is unacceptable for young patients. Therefore, some studies recommended more conservative treatment for example marsupialization of the cysts or decompression followed by secondary enucleation for young patients with large cysts (17). Because the epithelial islands and daughter cysts were not seen in the fibrous connective tissue in this case, the patient underwent treatment by enucleation and curettage of the jaw cysts and extraction of the impacted teeth. Two years and six months after the last surgical treatment, no recurrence of cysts in panoramic X-ray controls were detected.
The most common skin lesion of BCNS is cutaneous basal cell carcinoma (BCC). The relatively low frequency of skins in African-American may be returns the protective action of melanotic pigmentation from ultraviolet light (15). Epidemiological studies have revealed that sunlight is the main risk factor for basal cell carcinoma development. Early detection and management protocols were recently suggested in order to reduce death from cancer (18). Surgical excision, electrodessication and curettage are performed to treat small BCCs. Also, other treatments such as laser ablation, photodynamic therapy, and topical chemotherapy may be used in patients with multiple BCC (11). Palmar and plantar pits are specific signs of this syndrome (3). Fortunately, our case did not have cutaneous lesions.
Almost 70% of syndromic patients have various degrees of craniofacial anomaly. These anomalies contain frontal bossing, broad nasal root, maxillary hypoplasia and mandibular hyperplasia with variable prognathism, malocclusions, impaction and agenesis of teeth. Other skeletal abnormalities in BCNS patients include sprengel deformity, finger syndactyly, vertebral anomalies and bifid ribs (15).
In our case, malocclusion, impaction of teeth, syndactyly in both hands and bifid ribs were seen. This patient did not have the familial history of BCNS. If a family contains more than one affected individual, molecular methods of analysis can be undertaken. All the clinical manifestations of BCNS may not be observed in the patient with BSNC. In the case, two major criteria (OKC of the jaw and bifid rib) and one minor criteria (syndactylism) were present and that is adequate to establish syndromic patient.
Early diagnosis of malignant lesions can lead to faster treatment, better prognosis and decrease in the severity of maxillofacial destruction (1). The prognosis is related to the progress of skin cancers and other tumors with this syndrome (6). However, most patients with BCNS have a very good prognosis and their expectancy is commonly normal (1).
3.1. Conclusion
In conclusion, we must evaluate the patient with multiple odontogenic keratocysts and dental abnormalities for the risk of BCNS. We suggest clinico-radiographic evaluation for syndromic patients and long term follow up.