1. Background
The hippocampus may be one of the maladaptive plasticity sites after chronic opiate use (1), which is critical in the rewarding response (2) and drug-seeking behavior (3). It has been reported that chronic exposure to opiate could significantly decrease neurogenesis and produce dendritic atrophy or neurodegeneration, alter synaptic transmission in the hippocampus(1, 4). Attenuation of adult neurogenesis interferes with learning of a hippocampus-dependent task and expression of long-term potentiation (LTP) in vitro (1, 5), and increases a vulnerability to anxiety-like behaviors (6). In human subjects and in rodents, voluntary or forced exercises promote neurogenesis in the adult dentate gyrus (DG) (7) and brain-derived neurotrophic factor (BDNF) might have a potential role in exercise-induced neurogenesis, synaptic plasticity, and memory through its actions on the tyrosine kinase B (TrkB) receptor (8-14). We have recently demonstrated that voluntary exercise diminishes the severity of morphine dependency (15) and the anxiogenic-like behaviors in both morphine-dependent and withdrawn rats (16), enhances LTP in hippocampal DG area (17), and ameliorates the spatial memory deficits in morphine-dependent rats through TrkB receptor of BDNF (15).
2. Objectives
This study aimed to investigate whether voluntary exercise would reverse the attenuation of induced morphine dependence-induced hippocampal neuron number. If an effect of voluntary exercise was observed, the possible role of hippocampal BDNF in voluntary exercise-induced enhancement of the neuron number in morphine dependent rat was traced.
3. Materials and Methods
3.1. Animals and Dependency Induction
Adult male Wistar rats (mean weight, 220 ± 10 g) were obtained from breeding colony of Semnan University of Medical Sciences, Semnan, Iran. All rats were individually housed in cages in a 12-h light/dark cycle at 22°C to 24°C, with food and water ad libitum. All efforts were made to minimize the number of animals used and their suffering.
Animals received subcutaneous injection of morphine sulfate (10 mg/kg) twice a day at 12 hours intervals for ten days as described previously (15). Control rats received normal saline instead of morphine.
3.2. Exercise Paradigm
Each rat in exercise group had access to a running wheel (diameter, 34.5 cm; width, 9.5 cm) that was freely rotated against a resistance of 100 g. Each wheel was prepared with a magnetic switch, which was connected to a separate counter located outside the animal houseand monitored revolutions every hour. The sedentary rats were confined to the similar cages with no access to the wheels. The exercise groups were exposed to exercise during process of dependency-to-morphine induction for ten days. The cages were checked for food and water daily, and animals were weighed at first and tenth days of exercise, as described previously. Every rat had to run a minimum of 100 m each night (15).
3.3. Surgical Procedures and TrkB Receptors Blocking Protocol
3.3.1 Drugs
Recombinant human TrkB/Fc Chimera (TrkB IgG) was acquired in powder form (R and D System Inc, Minneapolis, Minnesota, the USA). This chimerical protein has been shown to be a highly potent and specific antagonist of BDNF action (18, 19). We used cytochrome C (Cyt C), obtained in powder from Sigma (St. Louis, Missouri, USA) as the control drug. Fluorescent latex microbeads, used as the vehicle to deliver drug directly into hippocampus (Lumafluor Corporation, Naples, Florida, USA). We prepared drugs and microspheres using methods described previously (15).
3.3.2. Animals and Preparation of Microbeads
To determine the effects of BDNF action blockade on the exercise-enhanced hippocampal neuron number in morphine dependency, rats were randomly allocated to the following eight groups of eight:
1) Saline-treated exercised group that received a Cyt C injection (Sal-Exc/Cyt C).
2) Saline-treated exercised group that received a TrkB-IgG injection (Sal-Exc/IgG).
3) Morphine-dependent exercised group that received a Cyt C injection (D-Exc/Cyt C).
4) Morphine-dependent exercised group that received a TrkB-IgG injection (D-Exc/IgG).
5) Saline-treated sedentary group that received a Cyt C injection (Sal-Sed/Cyt C).
6) Saline-treated sedentary group that received a TrkB-IgG injection (Sal-Sed/IgG).
7) Morphine-dependent sedentary group that received a Cyt C injection (D-Sed/Cyt C).
8) morphine-dependent sedentary group that received a TrkB-IgG injection (D-Sed/IgG).
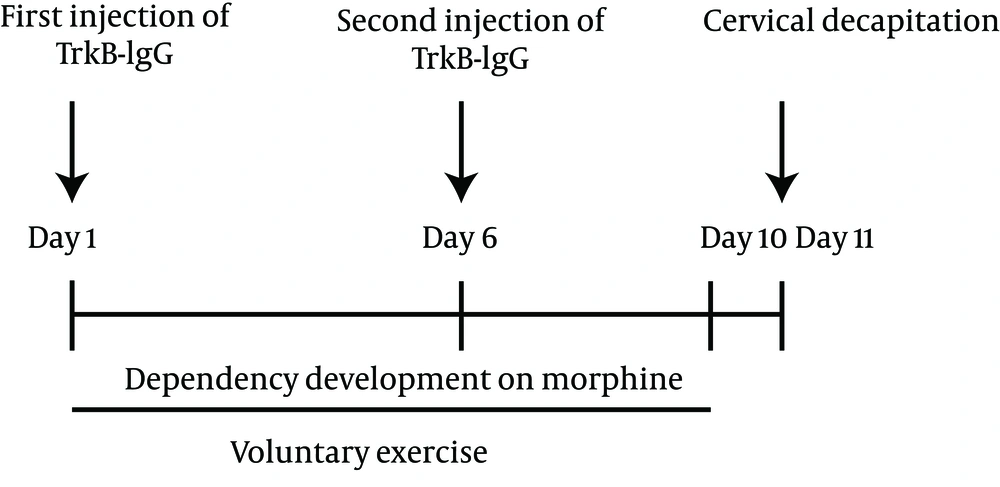
Two injections (TrkB-IgG or CytC) were done directly and bilaterally into the right and left hippocampus (-3.8 mm from Bregma, 2.6 mm from the midline toward left and right, and 3.7 mm vertically), by five-day interval in a ten-day running period (Figure 1). We prepared microspheres using methods described elsewhere. Each rat received injections of 2 µL of TrkB-IgG or Cyt C using a Hamilton syringe, over a period of 15 minute (15).
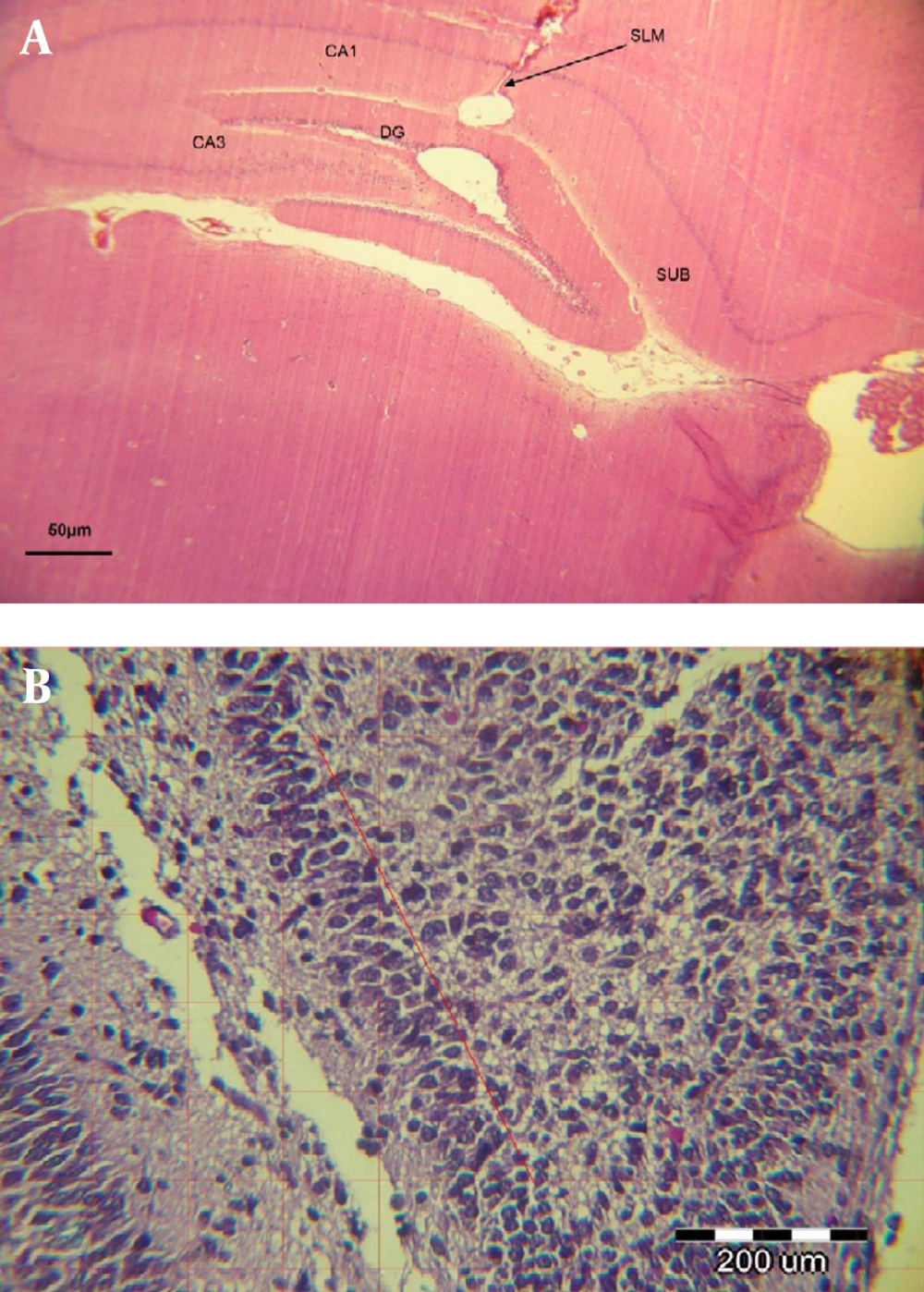
The proper microbead injection site was confirmed histologically (Figure 2A). Immediately after running exercise (on day 11), all rats were killed by decapitation. Immediately after decapitation, each brain was rapidly removed, and the two hemispheres were separated along the midline. Their left hemispheres were immerged in the fixing solution (4% paraformaldehyde in 0.1-M phosphate buffer, pH 7.4) and were used for neuron counting. Figure 2B represents the number of neurons counted in a section 1 × 1 mm of DG of the hippocampus.
3.4. Hippocampal Neuron Counting
As described earlier, left hemispheres of exercised and sedentary rats were used for neuron counting in different hippocampus regions. Left hippocampus were coronally cutat 5-µm thick sections and stained with cresyl fast violet (Nissl) (20) by an experimenter blinded to the study codes for each sample. Ten sections of each animal were used to neuron counting. Neurons in the DG (granule cell layer), CA1, CA3, and SUB (subiculum) were counted using a light microscope with a 40 × magnification (from the rostral most extreme of the hippocampus, at bregmaof 3.30 mm, to the caudal end, at bregmaof 5.80 mm), the counting frame of 100 × 100 µm, and the grid overlay of 200 × 200 µm. The optical fractionator method (Stereo Investigator; Micro Bright Field, Williston, Vermont, USA) was used to estimate the neuron number and the average number of the neurons was then calculated for each animal (21).
3.5. Statistical Analysis
Values were presented as the mean ± standard error of the mean (SEM). Two-way or three-way analyses of variance (ANOVA) were conducted, with repeated measures as required. Posthoc analyses included Tukey’s test. Statistical differences were considered significant at P < 0.05.
4. Results
4.1. Running Distance
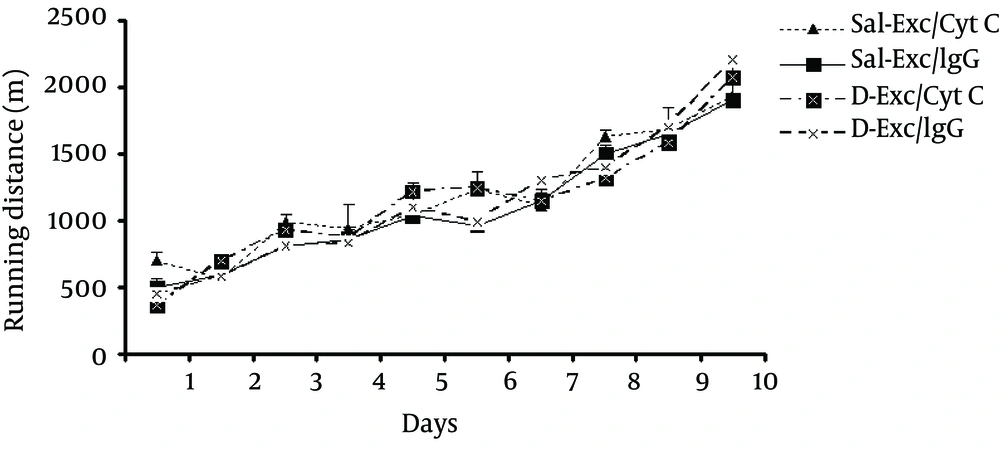
Our results showed that the average distance ran (m) at ten days voluntary exercise did not differ significantly (F3,25 = 0.7;P = 0.92) between exercising groups: Sal-Exc/CytC,11641.72 ± 250.9; Sal-Exc/IgG,10959 ± 349.31;D-Exc/Cyt C,11444.96 ± 287; and D-Exc/IgG,11388.58 ± 170.3 m. However, there was a significant effect of days (F9, 225 = 74, P = 0.002) while no significant interaction between both factors existed (F27, 225 = 0.798, P = 0.721) (Figure 3). In general, the running distance increased significantly as exercise days progressed.
4.2. Neuron Counting
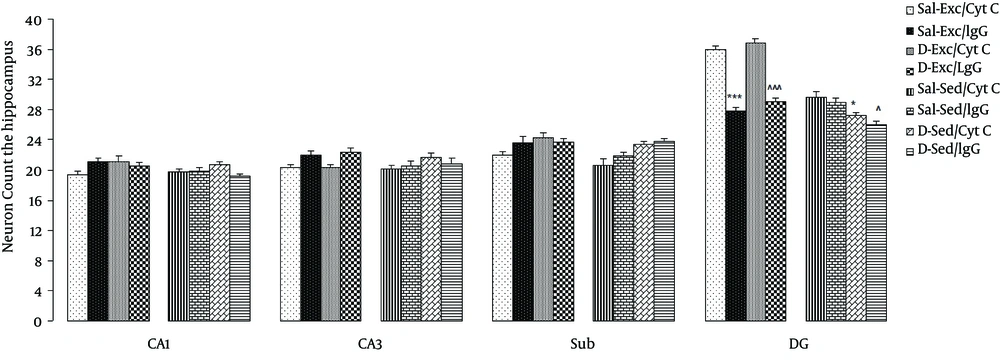
Data of the effect of exercise and BDNF blockage on the number of neuron in the hippocampus are shown in Figure 4. A three-way ANOVA (exercise × morphine × antibody) on neuron counts showed significant main effects of exercise (F1,56 = 18.85, P = 0.0001) and morphine (F1,56 = 5.42, P = 0.001), and a significant interaction between exercise and IgG (F1,56 = 3.8, P = 0.05). Significant differences were found only in the DG of the hippocampus (granular cell layer) (Figure 3), but not in the CA1, CA3, and subiculum regions of hippocampus.
Between group comparisons indicated that exercise groups (Sal-Exc/Cyt C and D-Exc/Cyt C) showed an increase in the number of neurons following ten-day exercise; moreover, blocking the BDNF action with TrkB-IgG fully inhibited this effect such that the number of neurons in DG of the Sal-Exc/IgG and D-Exc/IgG rats was significantly lower than those of the Sal-Exc/Cyt C and D-Exc/Cyt C rats were, respectively (P = 0.0001 for both).
Finally, chronic exposure to morphine itself more significantly decreased the number of DG neurons in the D-Sed/Cyt C and the D-Sed/IgG in comparison to the Sal-Sed/Cyt C and the Sal-Sed/IgG rats (P = 0.040 and P = 0.006, respectively).
Data are expressed as the mean ± SEM. exercise significantly increased the number of neuron in the dentate gyrus, but not in the CA1, CA3, and subiculum regions of hippocampus in Exc/Cyt C group. While, blocking the action of brain-derived neurotrophic factor significantly reduced the neuronal counting in the Exc/IgG Group. ***, the significant difference between Sal-Exc/Cyt C and Sal-Exc/IgG (P=0.000); ^^^,the significant difference between D-Exc/Cyt C and D-Exc/IgG (P = 0.000);*,the significant difference between D-Sed/Cyt C and Sal-Sed/Cyt C (P = 0.040); and ^,the significant difference between D-Sed/IgG and Sal-Sed/IgG (P = 0.006).
5. Discussion
Our results showed that chronic exposure to morphine decreased the number of neurons in the DG of the hippocampus in sedentary morphine dependent rats. This is consistent with previous results showing that chronic exposure to opiates such as morphine and heroin suppress neurogenesis (1, 4, 5, 9). In addition, we found that voluntary exercise enhanced the number of neurons in the DG of the hippocampus in both exercising dependent and independent groups. Blocking BDNF action significantly reduced this effect in both groups, indicating that BDNF mediates the exercise-induced enhancement of neuron numbers. This finding confirms the results of other studies showing the positive effects of exercise on neurogenesis (7, 12, 22, 23). Thus, exercise-induced enhancement in hippocampal BDNF expression and TrkB are necessary for BDNF-mediated effects on neuronal counting in exercising rats.
It seems that an interaction between BDNF and the number of neurons in the hippocampus may influence cognitive functions and plasticity in those with opiate dependency; increased levels of BDNF in exercising rats (15) in parallel with increasing the number of neurons in the hippocampus can ameliorate the spatial memory deficits in morphine-dependent rats. The finding that chronic use of opiates decreases the number of hippocampal neurons is in parallel with a poor performances of morphine-dependent rats on memory tasks (15). Voluntary exercise restored both the capacity of neuronal counting and the performances in the water maze test. Our finding is consistent with previous results showing that exercise enhances learning and memory capability through stimulating hippocampal neurogenesis leading to enhance learning and memory capability (9, 10, 12, 14, 18, 23, 24).
In line with our study, it was shown that BDNF action via the TrkB receptor is an important mediator between exercise and neural functions such as synaptic plasticity, neurogenesis, learning, and memory (7, 9-14, 25). The effects of exercise on behavioral and synaptic function are qualitatively similar to those of BDNF application (10).
Our study also showed that exercise only increases the number of neuron in the DG, but not in areas of CA1, CA3, and subiculum of morphine-dependent rats. In line with our study, it was shown that the hippocampal DG (as a source of neural stem cells) continues to generate new neurons throughout the lifespan, and physical activity also causes an increase in neurogenesis in the DG of the hippocampus, a brain area important for learning, memory (10), and synaptic plasticity (17). Our finding is consistent with previous results showing that exercise-associated changes in synaptic plasticity occurred in the same region where neurogenesis occurred by running, suggesting that newborn cells have a functional role in this process (9).
In our previous study, the morphine-induced BDNF protein levels was also higher in the sedentary morphine-dependent rats receiving TrkB-IgGs or Cyt C (15). Here, we showed that despite elevated levels of BDNF protein, the number of neurons in the hippocampal DG was lower in the sedentary morphine-dependent rats. One possible explanation is that chronic morphine administration may produce the BDNF gene polymorphism (26).
Our study demonstrates that voluntary exercise can ameliorate the decrement of hippocampal neuron number induced by morphine dependency through a BDNF-mediated mechanism. Thus, voluntary exercise could be a potential natural method for improving some of the deleterious behavioral, morphologic, and synaptic plasticity consequences of drug abuse.