1. Background
Uterine leiomyoma (fibroid) is a prevalent benign tumor observed in 20 to 25% of perimenopausal women and is one of the main reasons for female morbidity and hysterectomy (1, 2). Despite the high prevalence of uterine leiomyoma, its risk factors are not very well known (3, 4). Although estrogen and progesterone are known to promote tumor growth, chromosomal abnormalities, hormonal fluctuations, and growth and angiogenic factors have also been considered as etiological backgrounds of clonal proliferation of smooth muscle cells (5-7).
Vitamin D is an immunomodulatory steroid hormone and a strong anti-proliferator with a significant role in calcium homeostasis. Signaling 1,25(OH)2D3, the active form of Vitamin D, is exclusively mediated by vitamin D nuclear receptors that are found in significant volumes in endometrium and myometrium during menstrual cycle (8, 9). It has been known that an active form of vitamin D affects cellular proliferation, differentiation, cancerous invasion, and angiogenesis (10-13). Furthermore, vitamin D deficiency promotes cellular proliferation (13).
Initial in-vitro studies have shown that the active form of vitamin D effectively inhibits leiomyoma cell growth and it can be used to reduce uterine leiomyoma cell growth (14). In 2013, Lerchbaum and Rabe studied the relationship between vitamin D level and the risk of uterine leiomyoma (fibroids) (15). The results showed that the prevalence of uterine fibroids was about 32% lower in women with sufficient level of vitamin D than in those with vitamin D deficiency (15). In addition, based on the studies that have shown the destructive effects of 1,25(OH)2D3 on uterine leiomyoma, some researchers suggest the active form of Vitamin D might be used as a safe and non-invasive treatment (14). Treating vitamin D deficiency has been shown to inhibit leiomyoma cell growth and reduce the size of the tumor; therefore, vitamin D is considered as a new and safe treatment for uterine leiomyoma (16, 17). The goal of this study was to examine the effectiveness of vitamin D3 in the treatment of uterine leiomyoma in patients who also suffer from vitamin D deficiency.
2. Methods
Participants were selected from among women who visited the Gynecology Clinic of Amir-Almomenin Hospital affiliated to Semnan University of Medical Sciences for treatment of uterine leiomyoma. The inclusion criteria were: being between 20 and 45 years old, having at least one uterine leiomyoma 3 cm or larger in diameter, and having 1,25(OH)2D3 level of 30 ng/mL or lower (indicating Vitamin D deficiency). The exclusion criteria were the need for immediate surgery, history of kidney or liver dysfunctions, history of intestinal malabsorption, signs of malignancy in uterus, cervix, or ovaries, other pathological symptoms such as adnexal masses and/or endometrial polyps, uterine leiomyoma larger than 12 cm in diameter, use of hormonal medications (progesterone, contraceptives, Gonadotropin-releasing hormone agonists) within the last three months, pregnancy, and childbirth or breastfeeding during the last 6 months. Women who were trying to conceive were also excluded from the study.
Uterine leiomyoma was diagnosed by a radiologist. Sonographic images were carefully examined to determine the exact number, size, and location of fibroids, and to ensure there were no other uterine pathologies.
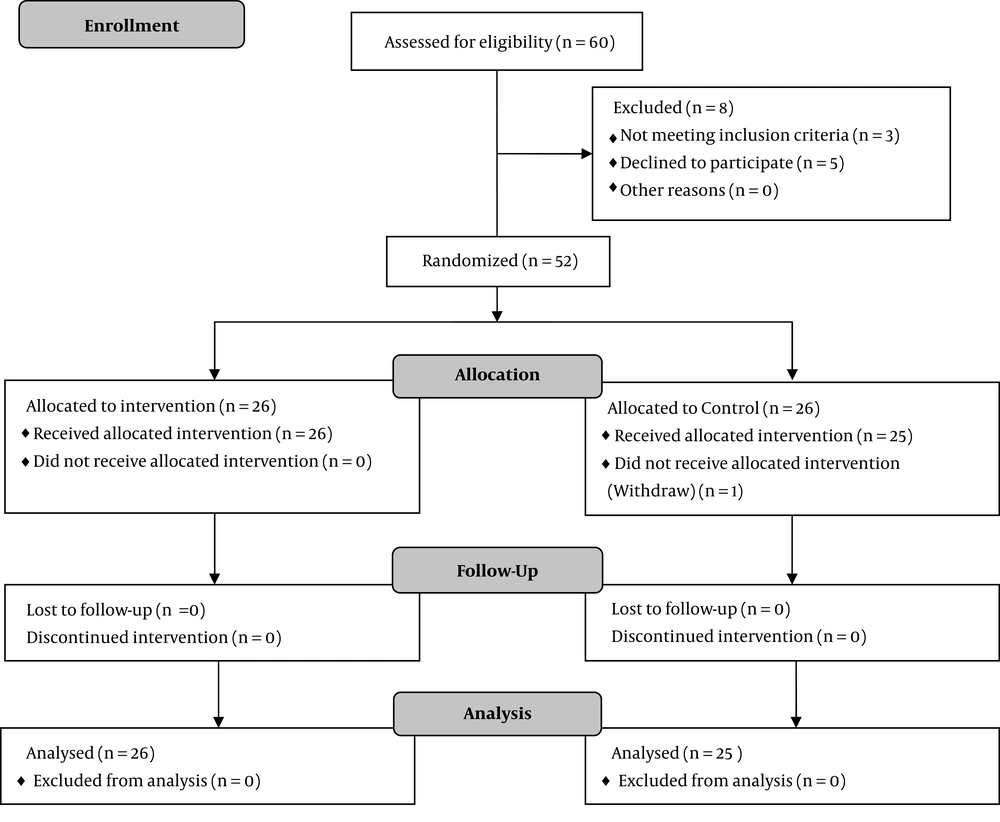
From those who were eligible, 52 women volunteered to participate in this study (Figure 1). Using the permuted block randomization method, the participants were divided into two equal groups: the intervention group and the control group. However, midway through the study, one of the participants from the control group withdrew from the study. Therefore, the study was completed with 26 women in the intervention and 25 women in the control group. This was a double-blind clinical trial, i.e., neither the investigators nor the participants were aware of group assignment.
All participants were informed about the experimental procedure and they signed written consent forms prior to participation in the study. All procedures were approved either by the research committee of Semnan University of Medical Sciences (Certification Number: 723) or by the Office of Research Ethics at Semnan University of Medical Sciences (Ethics Certification Number: 100). All procedures complied with Ethical Principles for Medical Research Involving Human Participants stated in the Declaration of Helsinki. The study protocol was registered in the IRCT database (Registration No.: IRCT2014121511019N4).
The intervention group received one 50000 IU Pearl vitamin D3 per week for 8 weeks. After eight weeks, the level of 25-hydroxyvitamin D3 was measured and if it was still below the normal level, the weekly treatments with 50000 IU Pearl vitamin D3 continued for another four weeks. However, if by the end of the first 8 weeks of treatment, the 25-hydroxyvitamin D3 level was back to normal, from that point on, the participant received only one treatment per month (i.e., one 50000 IU Pearl vitamin D3 per month).
The control group received weekly placebo treatments for eight weeks. Subsequently, they were tested for the level of 25-hydroxyvitamin D3, and placebo treatments continued for the control group on a monthly basis.
Both the vitamins and the placebo used in this study were manufactured by Zahravi pharmaceutical company. The packaging and appearance of the placebo and the vitamins were the same; the only difference was that the placebo did not contain the medicinal ingredients. Packages of placebo and Vitamin D were coded by Zahravi pharmaceutical company. At the beginning of the trial, we ensured that the participants in each group were given packages of the medication with the same code number (i.e., either the placebo or active medication). Only upon the completion of the trial, the codes were revealed to us and we could determine which group had received placebo and which one had received vitamin D. This was a double-blind study; hence, neither the researchers nor the participants were aware of the nature of their medication, i.e., placebo vs. vitamin D3, during the study.
The serum level of 25-hydroxyvitamin D3 was measured for all participants at the beginning of the study (baseline measurement), and again at two and four months into the experiment using 2 mL of each participant’s blood sample prepared by ELISA Kit (Monobind Inc., USA). Hemoglobin concentration level was also measured at the beginning of the experiment and again two and four months into the experiment using 1.5 mL of each participant’s blood sample. Cell Counter (Sysmex K1000, Japan) was used to measure the hemoglobin concentration level and ethylenediaminetetraacetic acid (EDTA) was used to stabilize the blood samples.
For all the participants, sonography was used to measure the size of leiomyoma before the study began and at four months into the experiment by the same radiologist. The radiologist was also blinded to group assignment and the nature of the treatment each participant received. Uterine leiomyoma volumes were calculated by the ellipsoid method and based on the following formula:
V = 0.5233 (D1 × D2 × D3)
in which D1, D2, and D3 represent the length, width, and sectional diameter of the fibroid (cm), respectively. In cases where there were multiple leiomyomas, the volumes of all leiomyomas were calculated and added up.
The pictorial blood assessment chart (PBAC) was used to evaluate the severity of bleeding during the menstrual period. PBAC was used to track the number of lightly, moderately, and heavily soiled pads and/or tampons participants had used during their menstrual period, and to record the passage of clots and episodes of flooding. Furthermore, a visual analogue scale (VAS) was used to evaluate pain (dysmenorrhea, dyspareunia, and pelvic pain) in the participants. This scale ranges from 0 to 10, with zero indicating the absence of a symptom and 10 indicating the most severe form of the symptom. Both PBAC and VAS were used at the beginning of the experiment and again at two and four months into the study. An obstetrician, also blinded to group assignment and the nature of the treatment each participant received, performed these evaluations.
Considering the shrinkage of uterine leiomyoma as the main outcome of the study, and assuming a standard deviation of 10% in the volume change, having 25 participants in each group was sufficient to give us 80% power in detecting a difference of at least 10% in the volume change between the two groups when using a two-tailed test with a significance level of 0.05 (4).
Statistical analyses were performed using SPSS version 14 software. The effect of vitamin D on the size of uterine leiomyoma and serum hemoglobin level was examined using Mann-Whitney U Test for independent samples. Fisher exact test was used to test the independence of two categorical variables. Wilcoxon Signed Ranks test was used to compare before and after measurements. Statistical significance was set at P < 0.05.
3. Results
Tables 1 and 2 summarize the characteristics of the participants. As the Tables show, there were no significant differences between the two groups in age, marital status, number of childbirths, baseline vitamin D level, PBAC index, baseline serum hemoglobin level, and VAS. Furthermore, the total volume of leiomyoma before the intervention was not different between the two groups (Table 3).
| Intervention Group (N = 26) | Control Group (N = 25) | P Valueb | |
|---|---|---|---|
| Age, y | 38.5 ± 5.5 (24 - 45) | 37.2 ± 5.9 (24 - 45) | 0.397 |
| Body mass index, kg/cm2 | 25.5 ± 2.1 (21 - 29) | 26.3 ± 2.5 (21 - 31) | 0.236 |
| Baseline vitamin D level, ng/mL | 12.1 ± 5.9 (4 - 25) | 14.2 ± 5.5 (5 - 25) | 0.186 |
| PBAC index | 37.6 ± 21.9 (15 - 90) | 30.5 ± 17.8 (8 - 72) | 0.217 |
| Baseline serum hemoglobin level, mg/dL | 11.7 ± 1.2 (9.5 - 14) | 11.6 ± 1.2 (9 - 13.8) | 0.941 |
| Visual analogue scale (VAS) | 6.6 ± 2.9 (0 - 10) | 7.4 ± 2.4 (3 - 10) | 0.353 |
| Marital status, No. (%) | 0.235c | ||
| Single | 0 | 2 (8) | |
| Married | 26 (100) | 23 (92) | |
| Number of childbirthsc | 2 (0 - 5) | 2 (0 - 4) | 0.339 |
Characteristics of the Participantsa
| Intervention Group (N = 26) | Control Group (N = 25) | P Valuea | |
|---|---|---|---|
| Vitamin D, ng/mL | |||
| Baseline | 12.1 ± 5.9 | 14.2 ± 5.5 | 0.174 |
| After two months | 34.3 ± 12.2 | 15.0 ± 7.5 | < 0.001 |
| After four months | 22.3 ± 8.1 | 13.0 ± 5.4 | 0.011 |
| PBAC | |||
| Baseline | 37.6 ± 21.9 | 30.5 ± 17.8 | 0.531 |
| After two months | 33.8 ± 22.6 | 27.8 ± 16.3 | 0.714 |
| After four months | 34.1 ± 23.3 | 28.1 ± 16.3 | 0.748 |
| VAS | |||
| Baseline | 6.6 ± 2.9 | 7.4 ± 2.4 | 0.188 |
| After two months | 5.6 ± 3.1 | 5.8 ± 2.9 | 0.455 |
| After four months | 5.2 ± 2.9 | 5.6 ± 2.8 | 0.423 |
The Mean and Standard Deviation of Vitamin D, PBAC, and VAS Measures for the Intervention and Control Groups Before Intervention (Baseline Measures) and at Two and Four Months Into the Treatment
The Median and Inter Quartile Range (IQR) of the Total Volume of Leiomyoma (cm3) for the Intervention (N = 26) and Control (N = 25) Groups at Baseline (Before Intervention) and at Four Months Into the Intervention
There was a significant difference between the two groups in changes in the level of vitamin D3 over the course of the study (P < 0.001). In the intervention group, vitamin D level increased after four months of treatment. In the control group, however, the level of vitamin D remained almost the same throughout the study (Table 2). It should be noted that in seven participants (26.9%), vitamin D did not reach its normal level (≥ 30 ng/mL) by the end of the study.
Ultrasound results showed significantly different changes in the size of leiomyoma between the two groups (P = 0.014). At the baseline, the median of the total volume of leiomyoma in the intervention and control groups was 731.8 and 440.5 cm3, respectively. After four months of treatment, the aforementioned values were 691.1 and 404.9 cm3. There was no significant difference between the two groups in the PBAC index for menstrual bleeding (P = 0.7) or in VAS index (P = 0.4) (Table 2).
In the intervention group, serum hemoglobin level was 11.7 mg/dL at the beginning of the experiment that reached 12.2 mg/dL after four months of treatment. In the control group, these measurements were 11.6 mg/dL and 11.7 mg/dL, respectively. There was no significant difference between the two groups in changes in the level of serum hemoglobin over the course of the experiment (P = 0.143).
After the completion of the study, three women from the intervention group (11.5%) and three women from the control group (12%) had a hysterectomy due to severe bleeding (P = 0.647).
4. Discussion
The purpose of the present study was to examine whether vitamin D administration is effective in the treatment of leiomyoma in patients who suffer from vitamin D deficiency. Our findings indicate that vitamin D3 supplement is effective in reducing the size of uterine leiomyoma in women who also suffer from vitamin D deficiency. These findings indicate that vitamin D not only inhibits leiomyoma cell growth, but also shrinks the tumor. The relationship between vitamin D deficiency and uterine leiomyoma has been examined in the previous studies, too (18-20). Vitamin D deficiency has been shown to be more common in patients with uterine leiomyoma (19). Baird et al. (18) has shown that women with an adequate level of vitamin D in their body have 32% less chance of uterine leiomyoma. Paffini and colleagues (19) have also shown that the risk of uterine leiomyoma is 2.2 times higher in women with vitamin D level of less than 10 ng/mL. Sabry and colleagues (20) showed a strong reverse relationship between vitamin D level and the severity of uterine leiomyoma; the lower the level of vitamin D, the more severe the uterine leiomyoma. These findings motivated the researchers to investigate the effect of vitamin D and its derivatives on uterine leiomyoma cells in vitro. Blauer et al. (14) showed that vitamin D inhibits leiomyoma cell growth. In another in-vitro study, Halder et al. (17) also showed that treatment with 1,25(OH)2D3 significantly reduced leiomyoma tumor size in Eker rats. To the best of our knowledge, the present study is the first clinical trial that has examined the effect of vitamin D on uterine leiomyoma in humans.
According to the guidelines provided by the Endocrinology Association, the human body’s vitamin D level should always be kept at its normal and physiologic level of 30 ng/mL. Individuals with vitamin D deficiency should take 50000 IU of vitamin D per week for eight weeks, and continue taking it on a monthly basis until the level of vitamin D in their body reaches the normal range. Interestingly, in our study despite four months of such treatment, in almost 27% of the patients, vitamin D was still below its normal physiologic level. If we had continued the treatment for a longer period, vitamin D might have eventually reached its normal physiologic level and leiomyoma might have shrunk even more and its resolution might have also reduced. However, time was an important limitation of the study. Although based on the current standard protocols, the administration of vitamin D is not a routine method for the treatment of leiomyoma and ethically, the control group was not deprived of an adequate treatment, we did not want to delay the initiation of treatment of vitamin D deficiency in this group for more than four months.
The side effects of uterine leiomyoma have significant negative impacts on patients’ quality of life. Abnormal uterine bleeding is one of the most common clinical symptoms of leiomyoma, and in most cases, it is the main reason for invasive treatments such as myomectomy and hysterectomy. In the present study, using vitamin D for leiomyoma treatment showed no effect on patients’ menstrual experience.
The relationship between vitamin D deficiency and the need for surgical treatment of leiomyoma is unclear, and the question remains as to whether treatment of vitamin D deficiency reduces the need for surgery and hence its side effects in these patients. Although the present study was not designed to answer the aforementioned question and the follow-up duration was not long enough to allow such investigation, we found that the prevalence of hysterectomy due to heavy menstrual bleeding was not different between the intervention and control groups. Following the completion of the study, three women from each group had a hysterectomy due to heavy bleeding (P = 0.647).
Patients with uterine leiomyoma often suffer from anemia due to their heavy menstrual bleeding. Therefore, it is expected that with the treatment of uterine leiomyoma with vitamin D, which results in reduced bleeding, the serum hemoglobin level increases. In the current study, changes in the serum hemoglobin level were greater for the intervention group than for the control group; however, the difference between the two groups was not significant. Considering that the serum hemoglobin level changes relatively slowly, it is possible that the study duration was not long enough to reveal the effect of changes in menstrual status on serum hemoglobin level. We suggest that future studies allow a longer follow-up time before measuring the changes in the serum hemoglobin level, or measure the amount of stored iron in the body.
Many researchers have examined the effect of vitamin D on the pathophysiology of uterine leiomyoma at the molecular level. Uterine leiomyoma is a tumor with a large volume of extra-cellular tissues such as collagen, proteoglycan, and fibronectin accompanied by increased smooth muscle cell proliferation (21). The main enzymes that cause degeneration of extra-cellular tissues are matrix metalloproteinases (MMPs) that clearly increase with vitamin D3 consumption (22). Furthermore, it has been shown that there are fewer vitamin D receptors in a leiomyoma lesion than in the surrounding uterine tissue (23). Numerous genetic polymorphisms have also been reported in genes of vitamin D receptors in leiomyoma tissue (24). Al-Hendy and colleagues (25) have shown that vitamin D3 reduces the expression of sex steroid receptors, especially estrogen, in uterine leiomyoma cells. Interestingly, estrogen has been known to play a critical role in the pathogenesis of uterine leiomyoma (5-7). These findings along with the direct effect of 1,25(OH)2D3 on inhibiting the growth (26, 27), reducing angiogenesis (28), and reducing metastasis of prostate, colon, breast, lung cancers, and melanoma (12) support the antitumoral effect of vitamin D.
Although in humans, vitamin D serum level remains the same for many years (29), the current level of vitamin D cannot be an accurate indicator of its level in the years prior to leiomyoma formation (19). On the other hand, many studies have reported an increased prevalence of vitamin D3 deficiency in women with uterine leiomyoma. However, the role of vitamin D in preventing uterine leiomyoma has not been studied yet, and longitudinal studies should be designed to examine such a role.
The present study was the first randomized double-blind clinical trial that examined the effect of treating vitamin D deficiency on uterine leiomyoma in a rather large group of patients. However, our study has its own limitations. First, due to patients’ reluctance toward transvaginal sonography and the high cost of the procedure, it was not possible to use transvaginal sonography to obtain a more accurate measurement of uterine leiomyomas. Another limitation of this study was its rather short follow-up duration that might have led to missing some of the long-term side effects of the treatment. It should also be noted that the present study was performed on patients who had both uterine leiomyoma and vitamin D deficiency. Whether the controlled use of vitamin D3 in patients with a physiological level of vitamin D also reduces the size of uterine leiomyoma requires further investigation.
4.1. Conclusion
We conclude that using vitamin D3 significantly reduces the total volume of uterine leiomyomas in patients who also suffer from vitamin D3 deficiency. Therefore, vitamin D3 can be considered as an effective adjuvant treatment for uterine leiomyoma in such patients.