1. Context
Facial nerve palsy (FNP) is the most common cranial nerve neuropathy caused by damage to the seventh cranial nerve and its motor nuclei (1, 2). There are several etiologies for FNP, with the most common one being acute facial palsy or Bell’s palsy (BP). About 70% of BP patients recover completely within six months (1). The aberrant regeneration of the nerve fibers after FNP may lead to several adverse consequences, including facial muscle weakness, contracture, hyperkinesia, atrophy, synkinesis, and asymmetry in the face muscles (3-5). Synkinesis is one of the sequelae of facial nerve paralysis, with its exact cause remaining to be identified. Facial synkinesis is an involuntary movement of a region of the face that occurs because of the voluntary movement of another region (6). Synkinesis occurs not only with voluntary movements, but also at rest during the blink reflex, which involves the cheek muscles, as well as in sleep when there is a continuous retraction of the upper and lower lips (7). Sequelae of FNP and synkinesis have a negative impact on the patient’s quality of life (8). Available treatments for reducing synkinesis include surgery (9), botulinum toxin A (BTX-A) injection, and rehabilitation (3, 10, 11). With the recent introduction of treatments such as BTX-A injection and neuromuscular retraining (NMR), surgical procedures are no longer widely used due to their complications (12).
BTX-A injection is one of the most widely-used treatments for synkinesis in medical centers around the globe that was first used in 1970 as a treatment for strabismus (13). This toxin is produced by Bacillus Clostridium botulinum under anaerobic conditions (14). The toxin can temporarily block presynaptic-acetylcholine release and lead to muscle paralysis (15-17). Despite its widespread use for reducing or resolving synkinesis and improving facial symmetry, there is some controversy among scholars about the effects, complications and allowed frequency, dose, and site of BTX-A injection (18-21).
Furthermore, several animal studies have attributed destructive effects to botulinum toxin, including decreased muscle mass and strength, decreased contractile material, and altered mRNA expression phenotype (22, 23). In addition, the results of some electromyographic studies indicate muscle atrophy persists long after injection; for example, masseter atrophy was not resolved even within 25 months (24). Other studies have reported a significant reduction in muscle amplitude remaining as long as 12 months after the injection (25), injection in the normal side (26) and other muscles (27). These changes can lead to muscle weakness in the involved side (28) and ultimately cause facial asymmetry during voluntary movements.
The aim of the therapeutic interventions for FNP is to improve facial symmetry by reducing or resolving synkinesis. In one study, BTX-A was found to have a significant effect more on the nerve endings of the synkinesis muscles than on the healthy muscles (29). This study seeks to answer the question of how effective BTX-A injection acts in improving facial symmetry through a review of the literature.
2. Evidence Acquisition
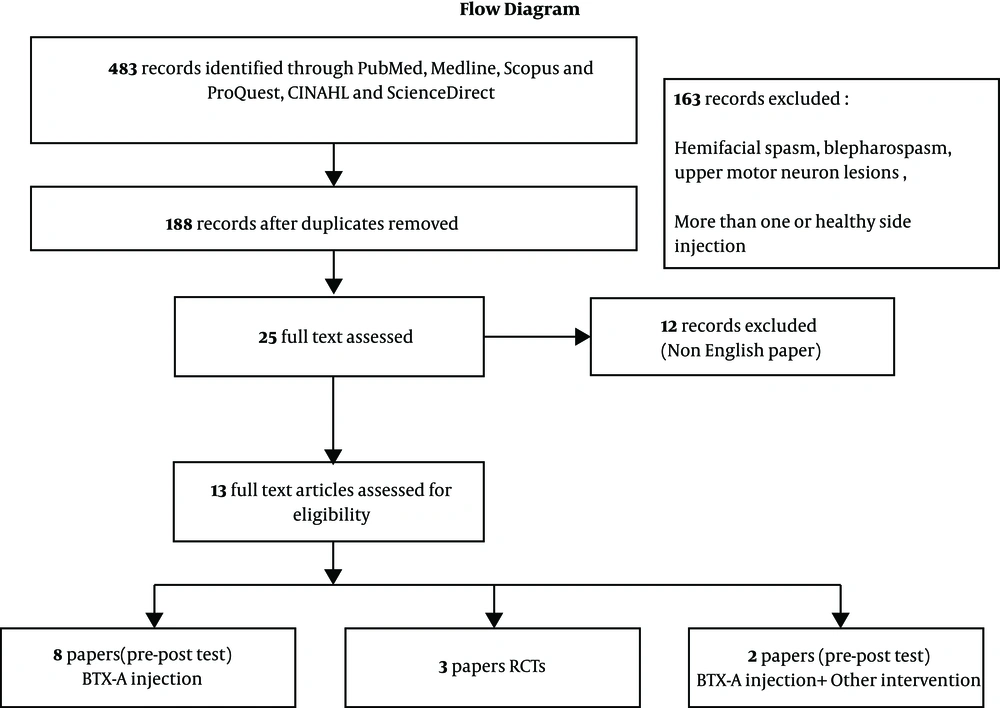
A computerized database search was carried out for clinical trials (randomized clinical trials and case studies) examining the effect of BTX-A injections indexed between 1970 and 2017 in PubMed, Medline, Scopus, ProQuest, CINHAL, and ScienceDirect, using the keywords ‘Botulinum toxin A’, ‘Facial nerve injury or facial nerve paralysis’, ‘Bell’s palsy’, ‘Synkinesis’ and ‘asymmetry’. The exclusion criteria were non-English articles, hemifacial spasm, blepharospasm, upper motor neuron lesions, and those in which the healthy side or both sides were injected (Figure 1).
3. Results
In total, 483 studies were retrieved and all were evaluated by two independent reviewers. Only were 13 eligible studies analyzed. According to the type of clinical studies, these articles were examined and divided into three groups:
(a) Clinical trials with no control group (pre/post-test with one intervention/case series)
(b) Clinical trials with no control group (per/post-test with milt-interventions/case series)
(c) Clinical trials with a control group (RCTs).
The first group comprised eight case series studies (Table 1). Four of these studies targeted ocular synkinesis and had injected BTX-A into the muscles around the eyes once or several times. In two other studies, the origin of synkinesis was the platysma and buccinators and BTX-A was injected into these muscles (30, 31).
| First Author, Year (Ref.) | Patients No. | Injections No. | Intervention and Injection | Outcomes Measurement | End of Treatment | Main Results | Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|
| Mountain, 1992 (32) | 4 | > One | orbicularis oculi | Photographs | - | All patients showed synkinesis improvement | BTX-A is an effective treatment in reducing synkinesis | There is no report of the objective evaluation |
| Roggenkamper, 1994 (33) | 23 | > One | orbicularis oculi | Patient’s satisfaction | - | Nearly all patients showed synkinesis improvement | BTX-A proves to be an effective treatment for involuntary lid closure | The measurement tool is not clear. The patient without improvement after injection refused further treatment |
| Boroojerdi, 1998 (18) | 10 | One | Orbicular oculi | A five-point scale, Videography | 4 weeks | Good to excellent (grades 3 and 4) effect over an average of six months after 91% of injections | BTX-A is an effective treatment in reducing synkinesis | |
| Chua, 2004 (34) | 5 | One | Pretarsal orbicularis | A three-point scale | - | All five patients had improvement of the synkinesis | Low-dose BTX-A is an effective treatment in aberrant facial nerve regeneration | |
| Ito, 2007 (19) | 11 | > One | Synkinesis area | - | - | In seven cases, synkinesis disappeared completely after three or fewer sessions of BTX-A injection | Facial synkinesis can be treated with a lower dose of BTX-A without side effects | Neither measurement tool nor the measurement method is clearly determined. |
| Filipo, 2012 (35) | 41 | One | Synkinesis area | FGS, Videotapes questionnaire | 1 month | All patients showed some improvement of the synkinesis | BTX-A injection is effective in the treatment of facial synkinesis and hyperkinesis | |
| Dall’Angelo, 2014 (30) | 45 | One | platysma | FGS, (specific platysma evaluation) | 18 days | Platysma synkinesis reduced. platysma symmetry at rest improved. The score of Sunnybrook facial grading scale increased. | BTX-A injection into the platysma muscle can increase the score of Sunnybrook scale by reduction of platysma synkinesis | |
| Wei, 2015 (31) | 42 | One | buccinators | Synkinesis (Synkinesis Assessment Questionnaire) + FGS | - | There were significant improvements in SAQ, as well as FGS. | Buccinator (Intraoral) BTX-A injection is effective in reducing synkinesis of the buccinator muscle | Time of re-assessment of patients is not determined. Just oral synkinesis is reviewed. |
Abbreviations: FGS, facial grading system.
In four of these studies, the facial grading scale (FGS) was used and improvements in its score were reported from 11% to 14%. To sum up, the studies in this group found that synkinesis disappeared or decreased following the given treatments, and some of them discussed symmetry, as well.
In the study conducted by Mountain et al. (32), the aim of the treatment was to reduce ocular synkinesis; for this purpose, BTX-A was injected into the orbicularis oculi of four patients and again repeated after seven months. The results were examined through photography and the patients reported a subjective improvement in their synkinesis.
In a retrospective study, Roggenkamper et al. (33) examined the results of 23 patients with ocular synkinesis who had been paralyzed for 12 months and injected BTX-A into their orbicularis oculi muscle. Almost all the patients reported an improvement in their synkinesis. After 19 weeks, some of the patients requested a second injection, as they were satisfied with the results of their first injection. In another study, Boroojerdi et al. (18) injected BTX-A into the orbicularis oculi of 10 patients with ocular synkinesis; six of these patients were videotaped before their injection and again two to four weeks after their injection, and the width of their affected lid was compared with that of their healthy side during movements. A five-point scale was used to evaluate the effectiveness of the treatment for synkinesis and all the patients ended up with a moderate to good improvement in their synkinesis.
Chua et al. (34) evaluated the effect of low-dose BTX-A injection in five patients with ocular synkinesis. In this study, the patients were examined for signs of reduction in their symptoms two to three months after the treatment. A three-point scale was used to evaluate the patients and the results showed that low-dose BTX-A injection is an effective treatment for reducing ocular synkinesis in patients with FNP.
Ito et al. (19) evaluated the effect of low-dose BTX-A injection on oral and ocular synkinesis in 11 patients with FNP. The signs of synkinesis disappeared in the patients two to three days after the injection. Another round of BTX-A injection was performed in order to maintain the effect of the injection and the interval between these two injections was 14.5 weeks. In seven patients, synkinesis disappeared completely after about three sessions of BTX-A injection. The results of this study suggested that low-dose BTX-A injection could be effective in reducing synkinesis without leaving any side-effects.
Filipo et al. (35) injected BTX-A to the upper, middle, and lower areas of the face in 41 patients with synkinesis and hyperkinesis and examined its outcomes after one month using photos, videotapes, a modified FGS, and the Synkinesis Assessment Questionnaire. The mean of the score obtained on the FGS increased from 56.0 before the treatment to 70.3 after the treatment, suggesting a statistically significant change (P < 0.001).
Dall’Angelo et al. (30) believe that facial synkinesis is due to platysma synkinesis and BTX-A injection into the platysma muscle can improve facial movements. They studied 69 patients with facial synkinesis, 45 of whom had platysma synkinesis and underwent BTX-A injections into their platysma muscles. The assessments were performed before and an average of 18 days after the treatment using FGS. A special platysma muscle evaluation was also performed on the platysma muscle symmetry at rest and platysma muscle synkinesis during voluntary movements as noted in the FGS. A subjective evaluation of pain, cramps, and aesthetic loss was also performed. The improvement of resting symmetry, the symmetry of the voluntary movements, and reduction of synkinesis were reported for all the patients. The median FGS increased from 50 before the treatment to 61 after the treatment, indicating an 11% improvement. The platysma muscle evaluation also showed an improvement in resting symmetry, as well as synkinesis in all the movements. The patients also reported a subjective improvement in their condition.
In a study by Wei et al. (31), 42 patients with buccinator synkinesis underwent BTX-A injection after six to 12 months of neuromuscular retraining. The researchers believed that the buccinator has an important role in facial expressions and thus, injected BTX-A into the subjects’ buccinator as well as the synkinesis muscles around their eyes and mouths. All the evaluations were performed before and after the treatment using FGS and the Synkinesis Assessment Questionnaire. Statistically significant improvements were observed in both scales. According to their results, the administration of BTX-A into the buccinator increased both scores and it was, therefore, appropriate treatment for patients with facial synkinesis.
The second group consisted of two clinical studies (Table 2). Exercise therapy was the primary treatment in both of these studies and BTX-A injection was used only as a complementary treatment. Final appraisals were made within 10 to 18 months. Navarrete Alvaro et al. (36) evaluated facial symmetry using the House-Brackmann and Azuma et al. (37) studied reductions in synkinesis and symmetry around the eyes only. The contributions of the injections or exercise therapy could not be distinguished, but both studies reported a reduction in synkinesis and an improvement in the symmetry was thus inferred.
| First Author, Year (Ref.) | Patients No. | Injections No. | Intervention and Injection | Outcomes Measurement | End of Treatment | Main Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Group II | |||||||
| Navarrete Alvaro, 2010 (36) | 48 | > one | BTX-A+ (NMR) | H-B, FGS, VAS | 12 - 18 months | Subjective efficacy of treatment was high | Rehabilitation treatment associated with BTX-A injection results in more patient satisfaction |
| Azuma, 2011 (37) | 13 | one | BTX-A + (mirror biofeedback) | Videotape, Ocular (asymmetry percentage of eye-opening width) | 10 months | The mean value of eye-opening percentage during movement increased significantly after the treatment period. | Mirror biofeedback after administration of a single dose of BTX-A is an effective and long-lasting treatment of established facial synkinesis |
| Group III | |||||||
| Borodic, 2004 (20) | 36 | one | BTX-A normal saline | (physician grading scale, measurement of palpebral asymmetry with facial movements), patients satisfaction (quality of life questionnaire), | 2 weeks | Subjective and objective improvements of patients were reported | BTX-A injection is an effective treatment of ocular synkinesis |
| Monini, 2011 (38) | 20 | one | BTX-A + (NMR) | FGS | 3 months | There were more improvements in facial synkinesis in patients who received BTX-A in addition to NMR | Rehabilitation of patients with facial synkinesis along with BTX-A injection is an effective treatment in patients with facial synkinesis |
| Pourmomeny, 2014 (11) | 34 | one | BTX-A +biofeedback biofeedback | FGS, videotape, Photoshop software | 4 months | There was a significant improvement in synkinesis in both groups, with no significant difference between the two groups | Biofeedback rehabilitation is as effective as biofeedback rehabilitation treatment along with BTX-A injection |
Abbreviations: BTX-A, Botulinum A toxin; FGS, facial grading system; H-B, house Brackmann; NMR, neuromuscular retraining; VAS, visual analog scale.
Navarrete Alvaro et al. (36) believed that NMR is an effective treatment for facial synkinesis and that BTX-A injections can be considered a complementary treatment. In this study, 48 patients with severe BP underwent BTX-A injection and NMR by a physiotherapist in hospital, as well as in the form of home exercise. The patients were assessed at the onset of their paralysis until 12-18 months after the injection and until they achieved complete recovery. The mean score in the final FGS was 56.9%.
Azuma et al. (37) studied 13 patients with ocular synkinesis who received a single dose of BTX-A injection, as well as 30 minutes of daily mirror biofeedback, for ten months. The patients’ facial movements in the frontal view were recorded using a videotape and through photographs. Their eye-opening symmetry was calculated by measuring the ratio of their interpalpebral space width in the affected eye to that of the normal eye in percentages. The results showed that mirror biofeedback after a single dose of BTX-A can be treated with lasting effects for the cases of established facial synkinesis.
The third group consisted of three randomized clinical trials (RCTs). Borodic et al. compared BTX-A injection and placebo only in the muscles around the eyes (20). The results of this study showed a significant reduction in ocular synkinesis. Monini et al. (38) and Pourmomeny et al. (39) obtained similar and comparable results although they reported different effects for BTX-A injection (Table 2).
In a study by Monini et al. (38), 20 patients with facial palsy undergoing Kabat physical rehabilitation for one year and with final House-Brackmann grade II and III after recovery were randomized into two groups. To assess the efficacy of BTX-A treatment in the final synkinesis score after NMR, the patients in the experimental group received one dose of BTX-A before NMR and those in the control group received NMR only. Both groups of patients were assessed once before and then 90 days after the treatment using the FGS. Significant differences were observed between the results of the treatments, as synkinesis improvement was greater in the experimental group than in the control group.
Borodic et al. (20) performed a double-blinded, placebo-controlled trial assessing the efficacy of BTX-A injection. Thirty-six subjects in this study received injections into the muscles around their eyes with BTX-A or normal saline in the case of the control group. The patients were assessed once before and then two weeks after the treatment using the Physicians Grading Scale and palpebral asymmetry during facial movements and the level of synkinesis was measured on a scale from 0 to 6 by videotaping. A questionnaire containing items on the problems faced due to facial synkinesis was also used to subjectively evaluate the patients. The data showed a significant improvement in ocular synkinesis while the controls showed no improvements.
Pourmomeny et al. (39) conducted a randomized trial on 34 patients who had received a combination of BTX-A and biofeedback or only biofeedback. At the start of the treatment, the patients in the experimental group received BTX-A treatment and those in the control group received normal saline. Both groups underwent biofeedback rehabilitation for four months. The patients were assessed before and four months after the treatment by using Photoshop, SFG, and videotaping. The results showed a reduction in facial synkinesis in both groups; however, there were no significant differences between the two groups in synkinesis reduction.
4. Discussion
The main goal of this study was to examine papers that have reported the effects of BTX-A on facial nerve damage. BTX-A injection was introduced in 1991 in papers published in German aiming to control synkinesis and improve facial symmetry (40). The most important complication of facial nerve damage is the asymmetry and weakness in the muscles damaged due to incomplete regeneration and synkinesis due to mal-regeneration or ephaptic transmission between adjacent axons or nuclear hyperexcitability. No matter the cause of the damage, those papers that had used BTX-A injections in the synkinesis muscles to reach facial movement symmetry were examined here. Except for special cases (such as the hyperlacrimation phenomenon), the patient’s expectation or the researcher’s goal was to achieve symmetry during the rest position and during active movement. Numerous studies even recommended BTX-A injection into the healthy side or both sides for achieving this goal (5, 41, 42). BTX-A injection cuts the chemical connection between the nerve endings and the muscular fibers and leaves part of the muscle paralyzed. After this connection is re-established at chemical synapses, movement or synkinesis is established once again. The effect of BTX-A has been reported to last for three to seven months (15, 21, 28, 29, 40, 43, 44), and re-injection is needed to continue the effectiveness. Nonetheless, as the body develops an antibody to botulinum toxin, its effectiveness will further decline following each injection (7, 16, 21, 45). Moreover, Dressler and Adib Saberi found that if BTX-A is injected into human body tissues for a prolonged period, permanent muscle atrophy becomes a possibility (46). In some of the studies conducted, atrophy and a considerable reduction in muscle amplitude were reported after BTX-A injection with no restoration for months, which is consistent with the hypothesis put forward by Dressler.
Yaraskavitch observed that BTX-A injection reduces the strength of the muscle adjacent to the site of injection (after four weeks of injection) in animals (27). Ansved et al. (47) and Girlanda et al. (48) proposed that atrophy could also be developed in the other muscles following BTX-A injection. In two studies, Fortuna showed that following three injections in the quadriceps of animals, atrophy was observed along with a significant decline in muscular strength, particularly in the third injection; what is interesting is that muscular strength declined even for the muscles that had not had injections (22, 23). Toffola et al. argued that synkinesis intensity gradually declines with repeated BTX-A injections because of the progressive atrophy of the muscle injected (28).
The results obtained by Couch et al. showed that as BTX-A is injected into the synkinesis muscles, the symmetry in the dynamic movements (standard expressions) also declines significantly after 35 days in addition to the reduction in synkinesis (49). Although various studies have been conducted to reduce or eliminate synkinesis, a few clinical trials have examined the effectiveness of this treatment. Moreover, there is no consensus about the injection dosage (31) and some researchers have even questioned the site of injection (34, 35, 50, 51). Dall’Angelo et al. (30) and Wei et al. (31) found platysma and buccinators to be the cause of synkinesis, respectively, and Filipo et al. found the injection technique and depth to affect the results (35). Ito et al. argued that the effect of lower doses is just as long-lasting as high doses or even more (19). Nonetheless, most of these studies have examined improvements in synkinesis rather than in symmetry.
Some studies even proposed BTX-A injection in the opposite side for creating symmetry (4, 42, 52) and found that it led the muscle function in the healthy side to the damaged side. In the present analysis, studies in which the injection had been made in the healthy side were excluded in order to identify the effects of BTX-A and its durability (for temporary or permanent paralysis) for symmetry. To increase the durability of BTX-A, some therapists increase its dosage, but this measure also increases the side effects and may result in systemic toxicity. Cakmak et al. argued that a comparison of hemifacial spasms and BP to the facial muscles shows significantly more complications after BTX-A injection, which could be due to the nature of the nerve damage in FNP (53). Nonetheless, it is not clear what will happen with injection into muscles that have previously been denervated and become regenerated.
In some studies, the goal was to reduce ocular synkinesis and increase symmetry, but these studies may not have been thorough because synkinesis can be observed in three upper and lower parts of the face (54). These studies reported the prevalence of synkinesis in the muscles around the mouth when raising the eyebrows or in the voluntary closing of the eyes as 85% - 89% while the prevalence of ocular synkinesis was reported as 82% (55).
The FGS is currently the most suitable instrument that can demonstrate three situations (facial symmetry at rest, five normal standard expressions, and the intensity of synkinesis) distinctly in the peripheral neuropathy and has a good validity (56). This instrument was used in only four studies and the maximum improvement was reported as 14%.
As for studies in which the researchers had not prescribed a regimen for correcting facial movements alongside their prescribed BTX-A injection, it is difficult to conceive of any patient who would not check his/her facial movements in the mirror and exercise. In other words, the question is how much mirror biofeedback has contributed to the improvements observed in these patients. In studies conducted by Navarrete Alvaro et al. and Azuma et al. BTX-A was introduced as a complementary treatment along with neuromuscular exercises. Navarrete Alvaro et al. believed that the efficacy of this rehabilitation was high and proposed muscle retraining as the essence of treatment. Azuma et al. assessed the percentage of ocular symmetry after injection and exercise therapy. Improvements in symmetry were reported in both studies, but the contribution of each of the two treatments could not be distinguished.
Only had three English RCTs been indexed (20, 38, 39). Borodic compared BTX-A injection and placebo only in the muscles around the eye and provided no reports of total face symmetry, whereas synkinesis may occur in the platysma muscle in addition to one half of the face (56). The patients in that study were appraised only in a two-week interval. Monini et al. and Pourmomeny et al. reported different effects for BTX-A; Monini et al.’s patients were selected from grade II and III only and were examined using the House-Brackmann scale, while Pourmomeny et al. reported BTX-A to not be effective after four months according to the three measurement instruments used and believed only muscle retraining to be effective. Mehdizadeh et al. argued that when neuromuscular exercise does not show a speedy recovery in some patients, BTX-A might be able to reduce the tension so that neuromuscular therapy can work faster (57). The limitations of our study were lack of access to non-English papers and lack of quantitative tools for measuring synkinesis directly.
5. Conclusions
BTX-A injections currently target temporary and permanent paralysis; in other words, they aim to remove muscular fibers receiving BTX-A. With repeated injections, this process continues in a defective cycle, and it is not clear to what extent the sprouting mechanism occurs afterward, and to what extent the phenomenon of neuroplasticity occurs in patients with neuromuscular exercise (whether the therapist has prescribed it or not). The 16-year clinical experience of researchers supports this claim; that is, the damage becomes fixed within several months to two years in patients who do not fully recover within the first few months, and after receiving several sets of BTX-A injection over one to two years, the patient eventually succumbed to the complications, unless receiving BTX-A becomes a motivation for NMR and the complications are controlled or reduced by neuroplasticity. Further biochemical and pathological animal studies or human clinical trials are recommended in order to achieve evidence-based results.