1. Background
Neck pain is a common disorder in industrial countries. Statistical analyses show that up to 37% of individuals with neck pain have developing and persistent symptoms such as reduced range of motion (1). Neck pain imposes a considerable economic burden on the health care system (1). Although there are many potential contributing factors to neck pain, myofascial trigger point (MTrP) is known as the main reason for it (2) and as an imperative cause of musculoskeletal dysfunction (3, 4). MTrP is the local, hyperirritable spot in the muscle fibers (5). Two types of MTrPs are (1) active MTrP (AMTrP) in patients with pain and (2) latent MTrP (LMTrP) in healthy individuals (6). AMTrP is considered as the cause of symptoms such as pain, restricted motion, and sympathetic response (7). LMTrP is not considered as the important source of pain. However, imposing pressure on LMTrP may lead to typically referred pains (6).
Any painful situation can cause some sort of changes in optimal muscles activation patterns during motion (8). Neck pain can change the coordination of cervical muscles and cause impaired proprioception in the neck (6). To have an ideal functionality, the joint requires a fine muscle activation pattern (9). The efficiency of muscle activation patterns can be evaluated by recording certain movements by electromyography (EMG) (10, 11).
According to the previous studies, scapular rotators, especially upper trapezius, generate dynamic stabilization of the scapula during upper limb elevation (12). The effects of scapular muscle injuries are investigated by the EMG and the onset of muscles recruitment pattern in this dysfunction is considered (8, 11, 13, 14). Some studies assessed the effect of MTrPs on muscles recruitment patterns (8, 15). Bohlooli et al. (3) investigated the onset of upper trapezius and serratus anterior muscles activations during rapid arm elevation in patients with upper trapezius LMTrP, and compared the results with experiments conducted on the control group. They concluded that there are significant differences in the onset of upper trapezius and serratus anterior muscles between LMTrP and control groups (3). One of the limitations of Bohloli’s study is the evaluation of a limited number of shoulder joint muscles.
Yassin et al. (16) examined the reaction time in postural muscles with AMTrP and LMTrP in the upper trapezius muscle. They showed that MTrPs are responsible for changing the reaction time. However, they did not investigate the muscles recruitment patterns in patients under study (16).
Postural muscles are important components in anticipatory postural control (10). Correct timing of muscles recruitment pattern is important during arm movement (17). Minimal changes in the performance and coordination of postural muscles may lead to dysfunctions that could compromise the performance of normal joints (3). Therefore, the primary objective of this study was to assess the recruitment pattern of certain cervical and shoulder muscles during arm flexion. Finally, we compared the recruitment patterns of cervical and shoulder muscles between patients with AMTrP in the upper trapezius and control groups.
The first hypothesis is that the active MTrPs may affect the onset of muscles activity and increase the latency of muscles. The second hypothesis is that the AMTrPs can change the muscles recruitment patterns in these subjects.
2. Methods
2.1. Subjects
15 women (aged 27.73 ± 3.43 years) as the control group participated in this study. They did not have any history of shoulder or neck pain. The subjects’ upper trapezius and other cervical muscles were checked to ensure the absence of LMTrPs in their muscles. Another 15 women participated as the patient group (aged 26.80 ± 2.67 years). The patient group had a history of recurrent pain produced by AMTrP in the upper trapezius muscle of the dominant hand for at least one year. The subjects were informed about the total experimental procedures and signed an informed consent form. All the processes were approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS, 92/D/130/297). Anthropometric characteristics of the participants in the control and AMTrP groups are listed in Table 1.
| Group | No. | Age, y | Weight, kg | Height, cm |
|---|---|---|---|---|
| Control | 15 | 27.73 ± 3.43 | 61.13 ± 6.55 | 162.00 ± 6.26 |
| AMTrP | 15 | 26.80 ± 2.67 | 57.07 ± 6.43 | 163.60 ± 5.94 |
Anthropometric Characteristics of Participants in the Control and AMTrP Groups
2.1.1. Identification of Active Myofascial Trigger Points
In order to identify the AMTrP, (1) the presence of a taut band, (2) the local twitch response, (3) the presence of at least one hypersensitive tender point in response to a 25 Newton/cm2 load on the taut band, (4) the spontaneous referral pain pattern (18, 19) and (5) the visual analogue scale (VAS) which was two or three centimeters in the assessment session (19) were investigated. The MTrPs were considered AMTrPs if all of the aforementioned criteria were met.
The subjects were excluded from this study if (1) there was a severe postural disorder, (2) they suffered from epilepsy, depression, migraine, and any mental health disorders, (3) there was a history of surgery in the shoulder and the cervical area six months prior to our experiments, (4) the treatment of MTrP was performed within a month prior to the experiments and (5) they had symptoms of arthrogenic pain, osteoarthritis, and radiculopathy of the cervical area and upper limb, and disorders of temporomandibular joint. In addition, we excluded the subjects who were in the period of the menstrual cycle (18, 20, 21).
In this study, upper trapezius 1 was the muscle with AMTrP in the patient group and upper trapezius 2 was the muscle free of AMTrP in the patient group. In the control group, both muscles were asymptomatic.
2.2. Collection and Processing of Data
2.2.1. Equipment
An eight-channel EMG system (Biometric Ltd., Gwent, UK) with CMRR > 96 dB at 60Hz, input impedance > 1012 Ω, the gain of 1000, the band-pass filter of 20 - 450 Hz, and sensitivity of 100 µv/div was used. Signals were acquired at a sampling frequency of 1000 Hz. The reusable surface electrodes (Biometrics Ltd., Gwent, UK) were used at fixed positions on the shaved and cleansed skin. The diameter of the electrodes was 10 mm and the inter-electrode distance was 20 mm. The ground electrode was attached to the wrist of the subjects and the reusable electrodes were placed on the belly of the muscles according to the SENIAM guideline. The electrode placements are listed in Table 2.
| Muscle | Location |
|---|---|
| Anterior deltoid | One finger width distal and anterior to the acromion |
| Cervical paraspinal | Level of the C4 |
| Lumbar paraspinal | Level of the iliac crest |
| Upper trapezius | Midway between acromioclavicular joint and C7 |
| Sternocleidomastoid | One-third of the distance from sternal notch to mastoid processes |
| Medial head of gastrocnemius | On the most prominent bulge of the muscle |
The Placement of the Electrodes
2.2.2. Procedure
First, the subject was asked to stand in an upright position on the force platform for 10 seconds with the hands fixed on both sides of the body. This position was repeated five times with 30 seconds as the trial interval. Then, the subject stood on the force platform while the shoulder was positioned at 60°, and the elbow was positioned in extended and pronated states. The main steps of the experiments were started while the displacement of the center of foot pressure was around ± 1 centimeters in the anterior-posterior direction (21).
Two different tones were used as a warning (S1) and response (S2) stimuli. A two-second time interval between S1 and S2 stimuli was introduced as the preparatory period. The duration and frequency of the S2 stimulus were 100 ms and 2 kHz, respectively. The intensity of S1 and S2 stimuli was set to 50 dB higher than the hearing threshold (20, 21).
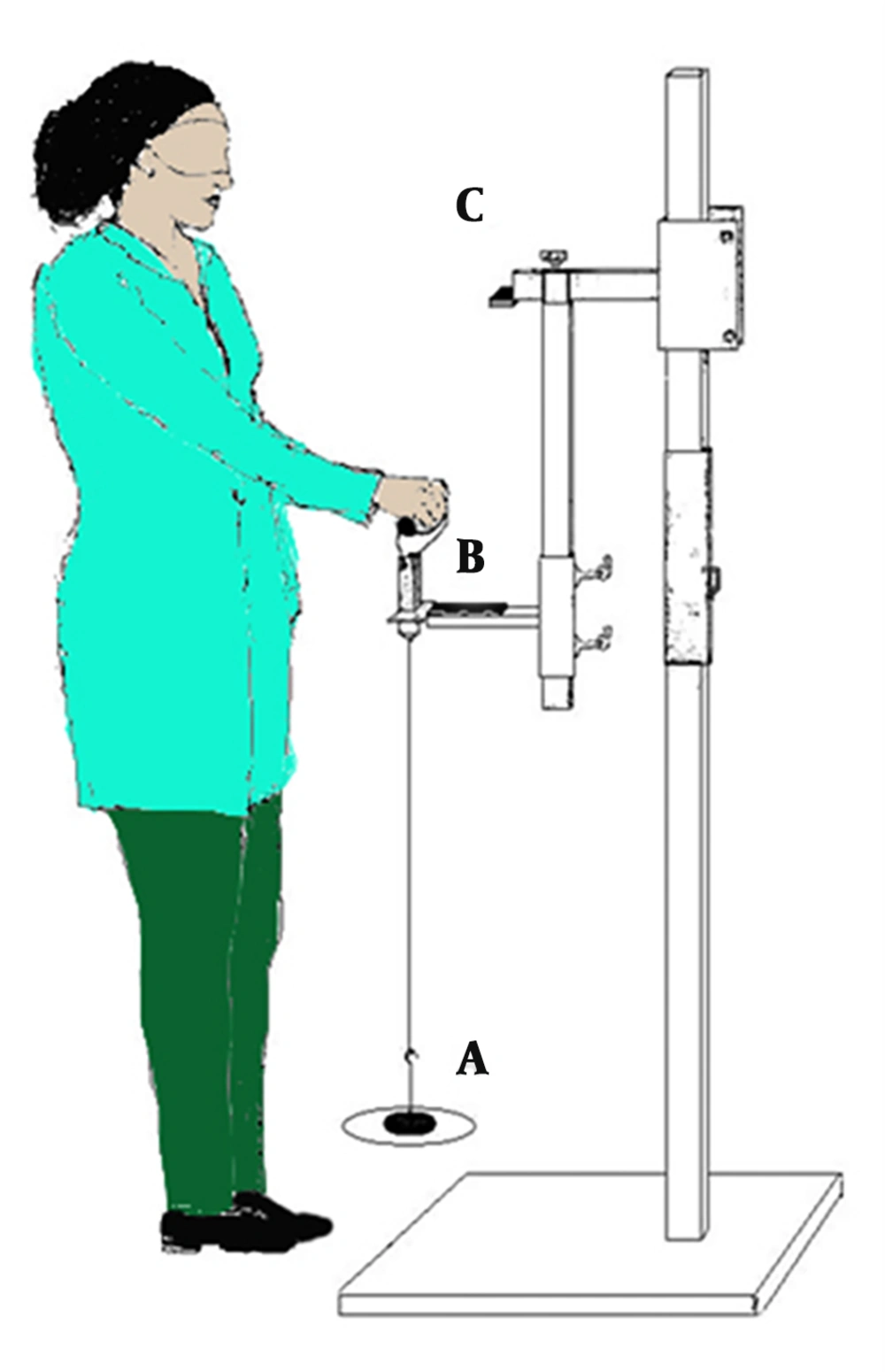
Figure 1 shows the schematic of the setup used for conducting the experiments. After the subjects stood for three seconds, S1 and S2 were activated. To minimize the response time to the onset of S2, the subject lifted a weight (item A in Figure 1) when flexing her shoulder as fast as possible. The weight was fixed at 2% of the subject’s body weight and was suspended from the lower segment of the designed system (20, 21). The subject held her hands at the shoulder level at about 90° for three seconds. Once the weight was elevated, a trigger was recorded by the SEMG signal. The range of arm motion was considered by using the initial angle (60°) of the shoulder flexion (item B in Figure 1) to the final angle (90°) of the shoulder flexion (item C in Figure 1) (21). An electrical sensor was placed on the subject’s shoulder to record and quantify the speed of shoulder motion. The initial shoulder flexion angle of the electrical sensor was 60°, which was displaced by an event marker on the SEMG signal. After the shoulder was flexed to 90°, the end of motion was detected by an electrical sensor synchronized with the SEMG (16). Anterior deltoid was investigated as the primary mover muscle for defining the onset of activity of muscles. The parameters chosen for the analyses were latencies and recruitment patterns of anterior deltoid, cervical paraspinal, lumbar paraspinal, upper trapezius 1 and 2, sternocleidomastoid, and the medial head of gastrocnemius muscles. The designed system is shown in Figure 1.
2.2.3. Electromyography
As a prime-mover muscle during the abduction, anterior deltoid was chosen in determining the onset of activity. An algorithm implemented in Matlab software (Version 7.6, 2008) was used for determining the onset of muscle activities. This algorithm runs a valid and reliable means for the onset of muscles activity (22). At the beginning of analysis to eliminate the movement artifacts, the raw EMG was filtered with a zero-phase shift and cut-off frequency of 30 Hz. After this step, the root means square (RMS) values were taken with a moving window of 100 ms. The onset of muscles activity was chosen when the first EMG signal rose above the mean value plus three times the standard deviation (SD) and persisted above this value during the subsequent 100-ms windows. This method for identifying the onset of muscle activity was according to the Moraes and Cools procedure.
2.3. Statistical Analysis
The Kolmogorov-Smirnov test indicated that data of the onset of muscles activity would satisfy the assumption of normality. Therefore, the independent t-tests were used for disclosing possible significant differences between control and AMTrPs groups in demographics. The variability of the onset of muscle activity in control and AMTrP groups was compared by a mixed between-within ANOVA test. SPSS version 17.0 (SPSS Inc. Chicago IL, USA) was used for conducting statistical calculations and statistical significance threshold was fixed to 0.05 for all tests.
3. Results
3.1. The onset of Muscles’ Activity
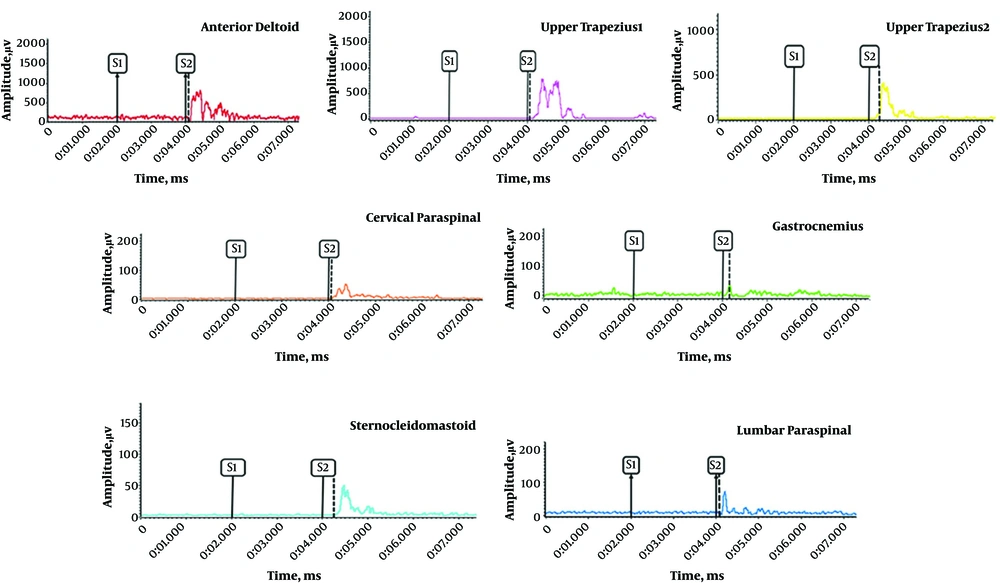
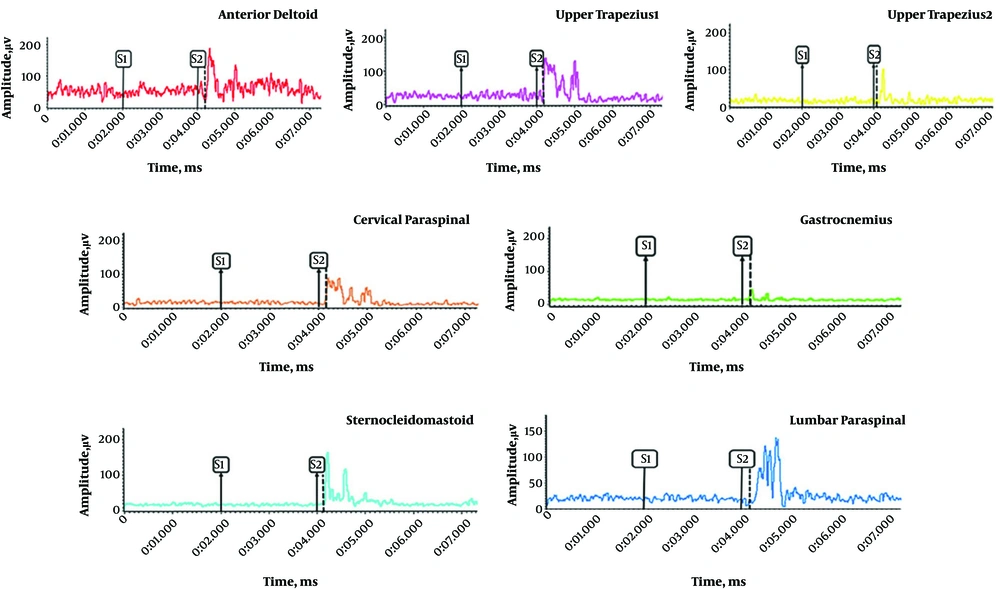
There was a significant difference between the control and AMTrP groups in the onset of activity of lumbar paraspinal muscle (P = 0.02), cervical paraspinal muscle (P = 0.01), upper trapezius 2 muscle (P = 0.04), upper trapezius 1 muscle (P = 0.05), and gastrocnemius muscle (P = 0.02). There was no significant difference between the control and AMTrP groups in the onset of activity of the sternocleidomastoid muscle (P = 0.67). The changes in the onset of activity of all muscles were relative to the anterior deltoid muscle onset (t = 0) during flexion of the shoulder. A sample of recorded EMG from muscles in the control and AMTrP groups is shown in Figures 2 and 3, respectability.
3.2. Muscles’ Recruitment Pattern
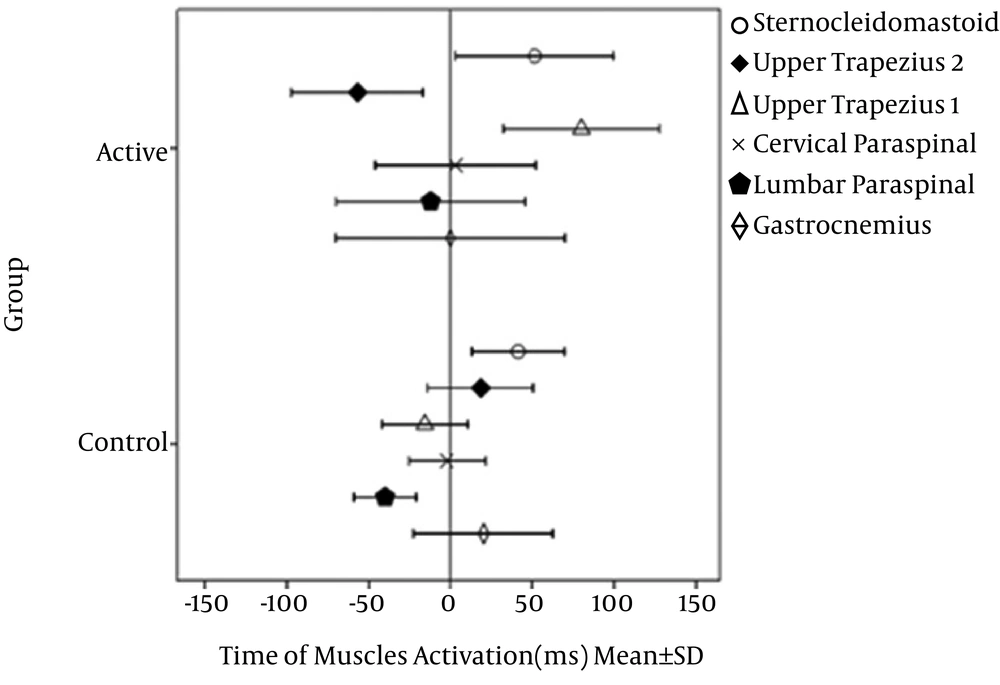
There was no significant interaction between the onset time and groups (Wilks Lambda = 0.82, F (2, 23) = 0.85, P = 0.55, partial eta squared = 0.18). There was a substantial main effect for the onset time (Wilks Lambda = 0.11, F (1, 23) = 29.04, P = 0.001, partial eta squared= 0.88). The main effect comparing the two groups was significant (F (1, 28) = 6. 97, P = 0.01, partial eta squared = 0.2). In the control group, the lumbar paraspinal muscle was activated first with the average of -42.08 ± 20.43, followed by the upper trapezius 1 muscle with the average of -20.31 ± 10.30 ms, the cervical paraspinal muscle with the average of -3.95 ± 2.67 ms, the gastrocnemius muscle with the average of 18.59 ± 12.32 ms, the sternocleidomastoid muscle with the average of 35.57 ± 24.12 ms and finally, the upper trapezius 2 muscle with the average of 74.16 ± 42.18 ms. In the AMTrP group, the upper trapezius 2 muscle was activated first with the average of -22.11 ± 17.23 ms, followed by the lumbar paraspinal muscle with the average of -12.13 ± 9.24 ms, the gastrocnemius muscle with the average of 0.11 ± 0.5 ms, the cervical paraspinal muscle with the average of 3.28 ± 2.54 ms, the sternocleidomastoid muscle with the average of 51.31 ± 36.43 ms, and the upper trapezius 1 muscle with the average of 79.91 ± 65.32 ms. The muscles’ recruitment patterns are shown in Figure 4 for both groups.
Muscles’ recruitment during flexion of the shoulder in the control and AMTrP groups. Anterior Deltoid onset (t = 0). Upper trapezius 1 is the muscle with AMTrP in the patient group. Upper trapezius 2 is the muscle-free AMTrP in the patient group. In the control group, both of the muscles are asymptomatic. AMTrP, active myofascial trigger point.
4. Discussion
The results demonstrated that AMTrPs caused a delay in the onset of muscles’ activity in the AMTrP group. This is in agreement with our hypothesis about changing the onset of muscles’ activity by AMTrP. The results also showed that the recruitment patterns of muscles were different between the two groups.
The findings of this study are supported by a study conducted by Maeda et al. (20). They showed that the postural muscles such as lumbar paraspinal and cervical paraspinal muscles are activated before upper trapezius during the flexion of the shoulder to play a role in the spinal stability (20, 21, 23). The activation of lumbar paraspinal and cervical paraspinal muscles leads to a postural control (24). On the other hand, lumbar paraspinal and cervical paraspinal muscles are activated before the start of the movement to create the control forces associated with rapid arm movements (25). The minimal change in the performance and coordination of postural muscles may lead to dysfunctions that could compromise the performance of normal joints (3). In the AMTrP group, the lumbar paraspinal and cervical paraspinal muscles were activated significantly later than the control group. This delay can compromise the functionality of the stability of the shoulder (13, 14). Neurophysiological (26), chemical (27, 28), and mechanical (28) changes may impair the recruitment of upper trapezius in patients with AMTrP.
The supplementary motor area, basal ganglia, and premotor area play a noticeable role in organizing the voluntary movements. This area also is involved in programming and planning of the movements (29-31). The motor programs are recruited and run by motor planning (29). Motor programs elect a certain group of muscles to control the correct timing of muscle activation (32). In other words, the motor programs define the appearances of movements, including the temporal organization and duration of muscles activity (33). Accordingly, the harmonized movements are dependent on the precise timing in the running and transferring of motor programs (30). In this study, we observed a delay in the onset of the activity of lumbar paraspinal, anterior deltoid, gastrocnemius, cervical paraspinal, upper trapezius 2, and upper trapezius 1 muscles in the AMTrP group. The delay in the onset of the activity of muscles in the AMTrP group leads to the modification of the recruitment patterns of the muscles. The altered pattern of muscles’ activity can cause an inefficient synergistic muscle activation that quickly generates adequate force in patients with AMTrPs (26). Sterling et al. (26) propose that the reflexes facilitated by pain can change the neuromuscular activation and recruitment of muscles.
Jacobs et al. (34, 35) claimed that patients with high irritability might encounter higher inputs, causing an increase in motor response and movement time. The first reason for the abnormal response to peripheral stimulation could be attributed to a disturbance in information processing (35). It is verified that the patients with AMTrPs have an impairment at the level of the limbic system, especially at the planning level (32). Another reason is the increased sympathetic response in patients with active myofascial trigger points that leads to increasing the cutaneous afferent inputs (31, 33, 36). Finally, the increased inputs affect gamma fusimotor in muscle spindle and cervical proprioception (1, 31, 34). Accordingly, cervical muscle tone will be increased to affect the onset of muscles activity. In order to have an effective motor planning, a combination of sensory inputs and clarification of information are essential (22). Difficulty in sensory processing may cause an unfortunate motor planning and then an ineffective postural preparation. This study indicates that AMTrP of the upper trapezius muscle may change the timing of muscles activation in the cervical spine and shoulder and finally, alter their recruitment patterns.
4.1. Conclusions
Based on these results, latency in the onset of muscles activity and altered muscles recruitment patterns were observed. These altered muscle recruitment patterns may lead to changes in motor control strategies and poor control of movement. Finally, these changes can cause a poor control of movement and increase the possibility of damage to the shoulder and cervical muscles in patients with AMTrP.
4.2. Limitations
All subjects in this study were female. Therefore, these results cannot be generalized to men with an active myofascial trigger. Electromyography is an indirect method for investigation of movement control. Thus, the direct investigation of the brain activity with electroencephalography synchronized with electromyography in subjects with myofascial trigger points is proposed.