1. Background
Nowadays, pesticides are used to deal with agricultural pests in order to increase crop production (1). Organophosphorus compounds are considered to be agricultural pesticides, and diazinon is an insecticide from the organophosphorus group, which enters the body via the skin, respiratory tract, or digestive tract, and in the liver and kidneys rapidly converts into active metabolite (2). In other words, diazinon is one of the most widely used organophosphate insecticides (OPIs) in agriculture and public health programs. Reactive oxygen species (ROS), including superoxide, hydrogen peroxide, hydroxyl radical, hydroxyl ion, and nitric oxide caused by OPIs may be involved in the toxicity of various pesticides (3). These compounds are responsible for around 100000 poisonings every year and are the third cause of poisoning and death (due to poisoning) in Iran (4). The greatest undesirable effect of diazinon poisoning was seen in the central nervous system (5). Organophosphorus compounds can react with macro-molecules or cell micro-molecules causing cell and genetic damages (6). Some researchers suggest that an increase in lipid peroxidation and the production of free radicals derived from the metabolism of organophosphorus pesticides are the main mechanisms of destruction in various cells and tissues of the body (7); consequently, the brain tissue appears to be one of the organs, which is affected by these toxins. In this regard, studies have shown that some organophosphorus products, by increasing the production of free radicals, can alter the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR) and increase the malondialdehyde (MDA) in various tissues (8). There are several strategies to improve the antioxidant enzymes, including sports activities and antioxidant supplementation.
Researchers have shown that physical activity can be considered a strategy to reduce oxidative stress and increase antioxidant enzymes. So far, various sports activities have been investigated in this regard, and most of them have been aerobic, swimming in water (9) or running on the treadmill (10). Resistance training (RT) today is a major component of physical fitness and rehabilitation programs (11). Some reports suggest that during exercise sessions, free radicals and ROSs may increase in response to RT (12, 13). The production of ROS is proportional to the oxygen used in oxidative phosphorylation and hence, individuals who participate in sports activities may increase the production of ROS levels in their bodies compared to those inactive. With intense breathing during exercise, with increasing body temperature and raising the levels of stress hormones, the production of free radicals increases (14). It is well-established that muscle contraction during exercise leads to elevated levels of ROS in the skeletal muscle. These highly reactive molecules have many deleterious effects, such as a reduction of force generation and increased muscle atrophy. From the discovery of exercise-induced oxidative stress several decades ago, evidence has been gathered that ROS produced during exercise also have positive effects by influencing cellular processes that lead to increased expression of antioxidants. These molecules are particularly elevated in regularly exercising the muscle to prevent negative effects of ROS by neutralizing the free radicals (15). On the other hand, today herbal medicines are used along with various methods of sports activities for improving the oxidative stress markers. Regarding the lower side effects of herbal-derived natural compounds, the use of herbal effective ingredients with anti-toxin and antioxidant potent has high clinical importance in treating poisoned people (16). One of these herbal effective ingredients is berberine chloride (BC).
Berberine chloride is a chemical compound, which is considered to be alkaloid salts and is found in many herbs such as grape, barberry, and turmeric. BC has beneficial effects on the health and functioning of the nervous system as well as can protect the neurons against damages (17). Clinical and preclinical studies have shown the beneficial effects of BC on diabetes, which is mainly attributed to its increased insulin secretion, B cell rebuilding, and antioxidant capacity (18). In addition, BC inhibits the activity of the acetylcholinesterase enzyme and plays an important role in metabolic syndrome (19). Finally, it has been reported that BC has beneficial effects on stimulating insulin secretion and modulating lipids (20).
2. Objectives
Owing to the lack of studies on dose-response effect of BC as well as the Interactive effects of RT and BC at the same time on oxidative stress markers in the cerebellum tissue, this study aimed to investigate the effect of RT with 2 mg/k and 15 mg/kg BC (15 mg/kg BC were used based on EC50 (50% effective concentration) of BC and 2 mg/kg BC as low effect dose BC) on oxidative stress markers in the cerebellum tissue of diazinon-poisoned rats; so the hypothesis of the present study is that RT with simultaneous consumption of BC have Interactive effects on improving the oxidative stress markers in the cerebellum tissue of diazinon-poisoned rats.
3. Methods
The study was approved by the Ethical Committee of Central Tehran Branch of Islamic Azad University. In this experimental study, 56 male Wistar rats weighing 250 ± 50 g were purchased from the Pasteur Institute of Iran and transferred to the Animal Health Laboratory of the Faculty of Neurosciences, Iranian University of Medical Sciences. During the research period, standard food plate (including crude protein: 23, crude fat: 3.5 - 4.5, crude fiber: 4 - 4.5, ash: maximum 10, calcium: 0.95 - 1, phosphorus 0.65 - 0.75, salt: 5 - 5.5, humidity: maximum 10, lysine: 1.15, methionine: 0.33, methionine + cysteine: 0.63, threonine: 0.72, and tryptophan: 0.25) and water were provided freely. All rats were kept in the standard situation (temperature of 23 ± 2°C, the light/dark cycle of 12:12, and the humidity of 45 to 55%). The rats were divided into seven groups (n = 8), including RT, BC2 (BC: 2 mg/kg), BC15 (BC: 15 mg/kg), RT + BC2, RT + BC15, diazinon, and control after seven days of adaptation to the laboratory environment.
3.1. Diazinon and BC
During the experiment, the groups of RT, BC2, BC15, RT + BC2, RT + BC15, and diazinon received 1.5 mg/kg diazinon (American Sigma Company, Code 454258-250 MG) for five times of the week for 4 weeks peritoneally. For injection of diazinon, at first, diazinon was diluted with 9% saline and every 20 microliters of diazinon was diluted in 1.5 mL of saline then, according to the weight of rats, each rat received 1.5 mg per kg of body weight (21).
The groups of BC2, BC15, RT + BC2, and RT + BC15 received BC (with specific doses) five times per week peritoneally. In the present study, BC was purchased from Sigma America Company (in the form of a 5 g yellow powder, Code B3251). Each 50 mg of BC diluted in 1 mL of saline (9% saline) and base on the weight of rats injected (22). Rats in the control group received saline for five times per week.
3.2. Resistance Training Protocol
Rats in RT groups performed climbing from a vertical ladder (Figure 1) (up to 1 meter with 26 steps) with a slope of 80 degrees with the weights attached to their tail, 3 sessions per week (23). Each RT session consisted of 2 sets of 6 repetitions, each requiring 8 to 12 active movements. The rest between each repetition was 60 seconds and between each set was 2 to 3 minutes. The training load started at 10% of the total body weight and reached 50% of the body weight by the end of the protocol (23).
3.3. Tissue Sampling
Forty-eight hours after the last training session and injection of BC and diazinon, rats were initially anesthetized using intraperitoneal (IP) injection of a combination of ketamine (30 - 50 mg/kg) and xylazine (3 - 5 mg/kg). Then the head of the animals was shaved and the skull was broken and cerebellum tissue was removed to measure the research variables. MDA (Cat No: ZB-MDA-48A/ ZB-MDA-96A; 0.1 µM sensitivity), SOD (Cat No: ZB-SOD-48A/ ZB-SOD-96A; 1 U/mL sensitivity), and CAT (Cat No: ZB-CAT-48A/ ZB-CAT-96A; (0.5 U/mL sensitivity) protein levels were measured using ELISA kits according to the manufacturer’s instructions (ZellBio, Germany).
3.4. Statistical Analysis
To analyze the normal distribution of the findings, Shapiro-Wilk test was used and to review the effects of RT and BC on research variables, independent sample t-test, two-way ANOVA, and Bonferroni’s post hoc tests were used by SPSS software (version 21) at the significance level of P < 0.05.
4. Results
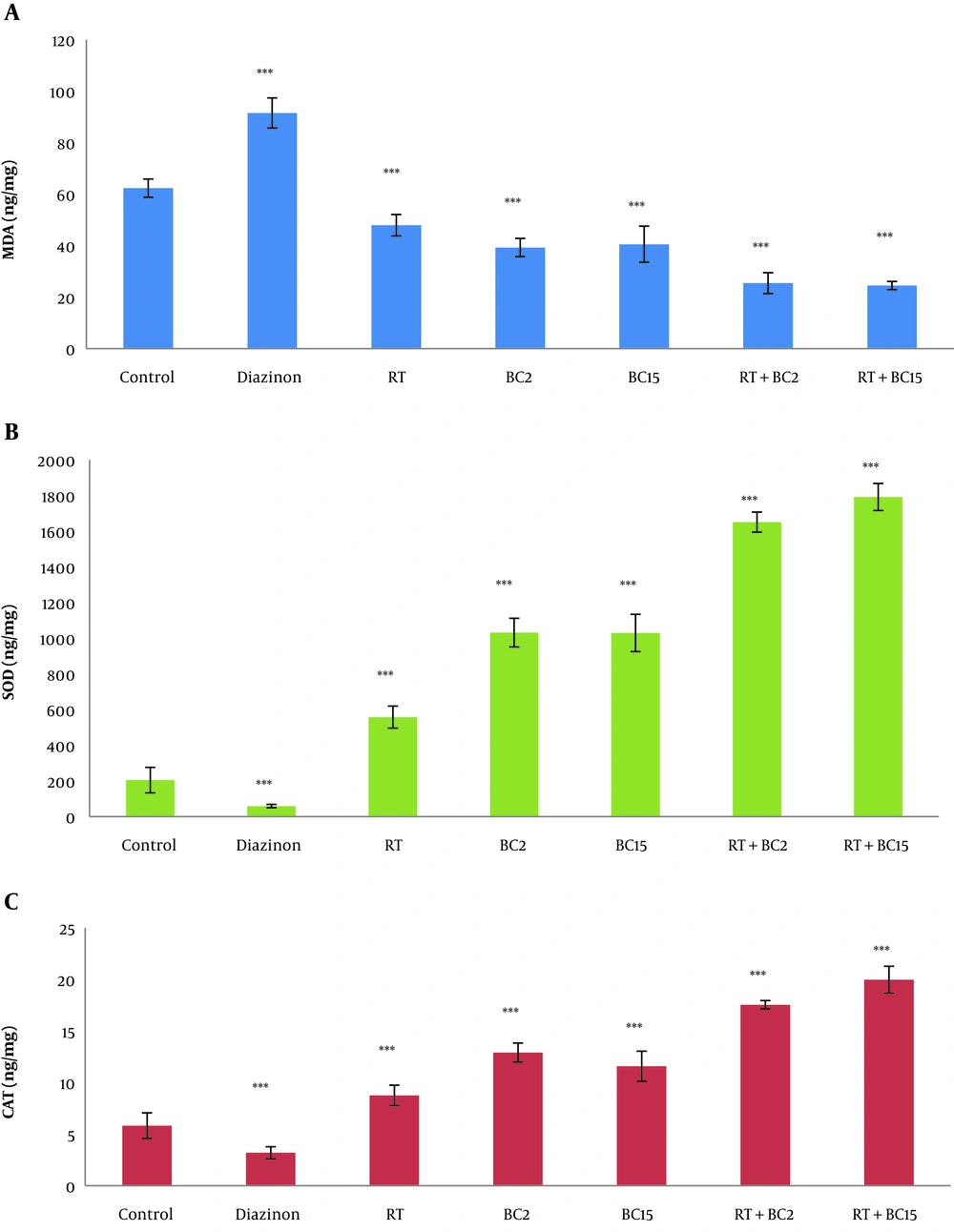
The protein levels of MDA, SO, and CAT in the seven study groups are presented in Table 1 and Figure 2A to 2C. The results of independent sample t-test showed that the MDA protein levels of cerebellum tissue (P = 0.001) in the diazinon group was significantly higher than the control group; whereas, SOD protein levels (P = 0.001) and CAT protein levels (P = 0.001) in the diazinon group were significantly lower than the control group.
| Control | Diazinon | RT | BC2 | BC15 | RT + BC2 | RT + BC15 | |
|---|---|---|---|---|---|---|---|
| MDA (Ng/MG) | 62.32 ± 3.54 | 91.52 ± 5.84 | 47.92 ± 4.16 | 39.26 ± 3.54 | 40.54 ± 7.02 | 25.42 ± 4.05 | 24.48 ± 1.59 |
| SOD (Ng/MG) | 204.53 ± 71.49 | 59.08 ± 9.36 | 557.87 ± 61.77 | 1031.45 ± 80.42 | 1029.64 ± 104.42 | 1651.04 ± 56.43 | 1791.94 ± 75.78 |
| CAT (Ng/MG) | 5.82 ± 1.24 | 3.18 ± 0.58 | 8.75 ± 0.98 | 12.90 ± 0.91 | 11.57 ± 1.46 | 17.54.040 | 19.96 ± 1.30 |
Abbreviations: BC2, 2 mg/kg berberine chloride consumption; BC15, 15 mg/kg berberine chloride consumption; CAT, catalase; MDA, malondialdehyde; RT, resistance training; RT + BC2, resistance training with 2 mg/kg berberine chloride consumption; RT + BC15, resistance training with 15 mg/kg berberine chloride consumption; SOD, superoxide dismutase.
aValues are expressed as mean ± SD.
Effect of RT and different doses of BC on oxidative stress indices in cerebellum tissue (A to C); A, MDA; B, SOD; C, CAT (mean ± SD). RT: resistance training; BC2: 2 mg/kg berberine chloride consumption; BC15: 15 mg/kg berberine chloride consumption; RT + BC2, resistance training with 2 mg/kg berberine chloride consumption; RT + BC15, resistance training with 15 mg/kg berberine chloride consumption; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase. *** Significant effect of diazinon on increase of MDA and reduction of SOD and CAT (P < 0.001). *** Significant effect of RT, BC2, BC15 and RT+ BC on reduction of MDA and increase of SOD and CAT (P < 0.001).
The results of two-way ANOVA test showed that RT (P = 0.001) and BC (P = 0.001) significantly decreased MDA protein levels of cerebellum tissue in diazinon-poisoned rats and RT and BC at the same time had Interactive effects in reducing the MDA protein levels (P = 0.001). Furthermore, RT (P = 0.001) and BC (P = 0.001) significantly increased the SOD protein levels of cerebellum tissue in diazinon-poisoned rats and RT simultaneously with BC had Interactive effects on increasing SOD protein levels (P = 0.001). Also, RT (P = 0.001) and BC (P = 0.001) significantly increased the CAT protein levels of cerebellum tissue in diazinon-poisoned rats and RT simultaneously with BC had Interactive effects on increasing CAT protein levels (P = 0.001).
The results of Bonferroni’s post hoc test showed that 2 mg/kg BC had a significant effect on the reduction of MDA (P = 0.001) and increase of SOD (P = 0.001), and CAT protein levels (P = 0.001). In this regard, 15 mg/kg BC showed a significant effect on the reduction of MDA (P = 0.001) and increase of SOD (P = 0.001) and CAT protein levels (P = 0.001). Further, 15 mg/kg BC rather than 2 mg/kg BC significantly increased SOD protein level (P = 0.02); However, 2 mg/kg BC and 15 mg/kg BC indicated the same effect on the reduction of MDA (P = 0.99) and increase of CAT protein levels (P = 0.40).
5. Discussion
The results of the present study showed that diazinon-induced oxidative stress in the cerebellum tissue of the rats caused a reduction in the activity of SOD and CAT, as well as an increase in the MDA levels; however, 4 weeks of RT and BC alone resulted in a significant increase in SOD and CAT and significant decrease in MDA also RT simultaneously with BC had Interactive effects on the decrease of MDA levels and increase of SOD and CAT levels.
Regarding RT, the studies have shown that low to moderate RT intensity improves the activity of antioxidant enzymes (increase in SOD, GSH, GPX, and CAT levels, while a reduction in MDA levels) in the skeletal muscle, which is consistent with the results of the present study (24). The findings of the present study are consistent with the study of Hamakawa et al. (25). It seems that adjusting the levels of antioxidant enzymes due to exercise depends on the high amount of oxidative stress in the skeletal muscle (25). Possibly, the increase in these enzymes following exercise will result in a further increase in lipid peroxidation and free radical production. Therefore, it can be said that a variable such as exercises may increase the antioxidant defense by likely increasing the activity of SOD and CAT due to increased detoxification capacity. Exercise with increased chronic oxidative stress, responses to activate internal antioxidant defense; therefore, RT is likely to reduce oxidative stress by increasing the antioxidant defense system, such as total antioxidant capacity. On the other hand, the reduction of blood flow during severe contractions contributes to the control of aerobic metabolism in the production of free radicals (26). High levels of calcium accumulation due to severe contractions, which usually cause damage, increase the production of ROS and possibly increase the capacity of the antioxidant system in response to the increased production of ROS; this is one of the mechanisms of compensating and adapting of the body to the oxidative stress (27). The results of the present study are consistent with the study of Albasser et al., which implies the role of RT on control of oxidative stress markers and prevention of lipid peroxidation as well as membrane vulnerability (28). It seems that the effect of training on the reduction of oxidative stress markers is associated with other protective mechanisms of training, including the increase in neurotrophic factors such as brain-derived neurotrophic factor and the relative improvement of brain blood flow during ischemia (29).
The findings of the present study indicate the effect of BC on the prevention of oxidative damage in the cerebellum tissue of diazinon poisoned-rats. Although 2 mg/kg and 15 mg/kg BC showed the same effects on MDA level and CAT, 15 mg/kg BC rather than 2 mg/kg BC significantly increased the SOD level. Berberine chloride has been reported to act as a direct antioxidant and an indirect deleterious agent in the body, neutralizing free radicals and protecting the body from oxidative stress by increasing the production of SOD, CAT, GSH, and GPX (30). In line with the present study, previous studies have shown that BC significantly reduced lipid peroxidation products with its antioxidant properties (17), thus BC prevents the damage to antioxidant enzymes against oxygen radicals and hydrogen peroxide. It has been shown that BC reduces the damage caused by oxidation in the brain (16). However, the antioxidant effects of BC were different in previous studies (20), which the main reasons may be as a result of selected tissue, injected dose, as well as laboratory methods for measuring the concentrations.
Concerning interaction effects, the results of the present study showed that four weeks of RT simultaneously with BC had Interactive effects on the increase in SOD and CAT levels and decrease in MDA levels in the cerebellum tissue of diazinon-poisoned rats. It seems that in the present study, BC combination with RT with synergist effects led to better and more improvement of antioxidant defense and lipid peroxidation markers. Therefore, given the fact that individuals are exposed daily to diazinon organophosphorus via agricultural and livestock products and even drinking water, it seems by using BC and performing RT may make desirable changes in lipid peroxidation and improve the anti-oxidation defense. It should be also noted that in this study, there were some limitations such as lack of control of calories in all groups as well as use the different methods of measurement such as real-time PCR and western blot. Thus it is proposed that future studies will investigate the different doses of diazinon and BC, in different tissues, and consideration of other methods of measurement such as western blot to measure the oxidative stress markers.
5.1. Conclusions
It seems that the combination of RT and BC have Interactive effects on the improvement of oxidative stress markers in the cerebellum tissue of diazinon-poisoned rats. Therefore, given that individuals are exposed to diazinon on a daily basis through agricultural and livestock products and even drinking water, it appears that there is a possibility of desirable changes in lipid peroxidation and improved antioxidant defense by consumption of BC (as an herbal antioxidant) and RT.