1. Background
Insufficiency of insulin secretion, insulin action, or both that cause chronic hyperglycemia, lead to diabetes mellitus as the main endocrine disorder (1). This pathological situation drives to the activation of several cellular pathways such as inflammation, apoptosis, and oxidative stress in renal or other tissue (2, 3).
Death of cells is a common issue in diseases. For diseases with an irreversible functional loss in the whole organs followed by the death of individual cells and ultimately mortality, a basic therapeutic goal is the prevention of cell death (4). Approximately, human adults lose 50 - 70 billion cells each day due to apoptosis. In a variety of disease states, dysregulation of cell death by defective or excessive apoptosis is implicated, although this programmed cell death is clearly necessary to maintain the health of the organism (5).
Apoptosis causes changes in diabetic renal structure and function by activating caspases (6). Usually, there is an association between the caspases with the induction of cellular apoptosis in response to signals of cell death, and reactive oxygen species (ROS)-mediated oxidative stress (7, 8). There are two extrinsic and intrinsic pathways of apoptosis that caspases are central to both apoptotic pathways (9). In diabetic kidneys, the mitochondrial pathway or the intrinsic pathway is apparently important (10). In the intrinsic pathway, cytochrome c is released, and caspase-3 and -9 are sequentially activated (10). Members of the Bcl-2 family of proteins have a significant role in controlling apoptosis. Mitochondrial outer membrane permeability is governed by Bcl-2 family proteins and this can be either anti-apoptotic or pro-apoptotic (e.g., Bcl-2/Bax) that can be induced by a variety of physiological and pathological stimuli (4).
For decades, it is observed that diet along with exercise training (ET) and medication can be considered as a cornerstone of diabetes management (11). Nowadays, it is well known that aerobic exercise improves glycemic control and reduces mortality due to cardiovascular diseases in patients with diabetes (11). Ghosh et al., demonstrated that moderate exercise can inhibit the progression of early diabetic nephropathy, independent of hyperglycemia. They stated that ET through a reduction in caspase-3 and caspase-8 activities, oxidative damage, and improvements in superoxide dismutase (SOD) expression has beneficial effects on diabetic kidney disease (6).
On the other hand, it is a long time that plants are the sources of various medicines as an effective strategy for prevention and therapy (12). Berberine chloride (BC) is a plant alkaloid, present in many medicinal plants, which has many pharmacological activities (12). Chandirasegaran et al., showed that anti-apoptotic (Bcl-2) protein increased and pro-apoptotic (Bax) protein decreased in the liver of streptozotocin (STZ)-induced diabetic rats treated with BC (13). Kumas et al., stated that different doses of berberine (100 and 150 mg) may be used as a potential antioxidant, anti-apoptotic, and anti-inflammatory agent in diabetic rats (14). However, the molecular mechanism underlying different doses of BC, in the renal tissue of diabetic rats, cell protection, and growth are not identified clearly.
2. Objectives
The current study aimed at investigating the inhibition of apoptosis induced by aerobic exercise training and supplementation with different doses of BC in renal tissue of STZ-induced diabetic rats.
3. Methods
3.1. Animals and Diabetes Induction
The current experimental study was conducted on 64 male Wistar rats (aged 8 - 10 weeks, weighing 240 - 280 g, obtained from Pasteur Institute of Tehran, Iran) maintained at 24 ± 1°C with a regular light-dark cycle (lights on from 8:00 a.m. to 8:00 p.m) in standard polycarbonate cages (three rat/cage) with free access to standard rodent chow and drinking water. The rats were randomly assigned into two normal and diabetic groups and then the normal group was divided into two subgroups of eight as the normal control (NC) and normal exercise training (NET), and the diabetic group was divided into six subgroups of eight as diabetic control (DC), diabetic exercise training (DET), diabetic BC15 (DBC 15 mg/kg), diabetic BC30 (DBC 30 mg/kg), diabetic BC15+ET (DBC15+ET), and diabetic BC30+ET (DBC30+ET). The study protocol was approved by the Ethics Committee of Sport Sciences Research Institute of Iran (code: IR.SSRC.REC.1398.059).
Single-dose intraperitoneal injection of 60 mg/kg body weight of STZ (Sigma-Aldrich, USA) in a buffer (0.1 M citrate buffer, pH 4.5) was administrated to induce diabetes in rats (15). An equivalent volume of normal saline was injected into two non-diabetic groups. From the beginning to 48 hours after induction, to prevent the drug-induced hypoglycemia, drinking a 5% glucose solution was allowed to the STZ-treated rats (13). After three days of STZ injection, glucose level of blood samples collected via tail vein puncture was measured (Glucocard-01, ARKRAY, Japan). Blood glucose level ≥ 300 mg/dL was considered as the cutoff point for diabetes diagnosis in the rats (16).
3.2. Aerobic Exercise Training Protocol
Rats were acclimatized to the treadmill running at 4 - 5 m/minute for 5 - 10 minutes over five days. The aerobic exercise training protocol involved in treadmill running for six weeks (3 d/wk, 50% - 55% of VO2max in diabetic rats) (Table 1) (16, 17). The ET program was performed based on loading principle and exercise intensity was 50% - 55% of VO2max in the last two weeks. Each ET session consisted of a five-minute warm-up and a five-minute cooling down at a speed of 4 - 5 m/minute, added to the main training time. Speed and duration of training gradually increased according to Table 1.
| Week | ||||||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
| Speed (m/min) | 10 | 10 | 14 - 15 | 14 - 15 | 17 - 18 | 17 - 18 |
| Duration (min) | 10 | 20 | 20 | 30 | 30 | 40 |
3.3. Berberine Chloride Supplementation
BC supplement (Sigma Aldrich, USA) was administrated every day via gavage. Diabetic rats were treated with 15 and 30 mg/kg of BC (12) and at the end of each week, after weighting the rats, the amount of solution was calculated based on the weight of rats. BC doses of 15 and 30 mg/kg were completely dissolved in 0.3 and 0.6 mL of normal saline, respectively.
3.4. Tissue Preparation and Determination of Caspase-3, BCL-2, and Bax Protein Levels
The rats were anesthetized 48 hours after the last ET session and BC consumption following 12 hours fasting by intraperitoneal injection of ketamine/xylazine (30 - 50/3 - 5 mg/kg). The renal tissue was quickly removed, frozen in liquid nitrogen immediately, and then stored at -80°C. To homogenize the tissue, phosphate-buffered saline (PBS) (pH 7.4, 100 mM) was utilized as a homogenization buffer. For this purpose, the sample was thawed at room temperature and then PBS was added to homogenize the sample thoroughly by a homogenizer. When the tissue was homogenized with lysis solution and total protein was extracted (Bradford Protein Assay Kit, ZellBio GmbH, Ulm, Germany); then, caspase-3 (intra-assay: Cv% < 12; sensitive: 0.01 ng/mL), Bcl-2 (intra-assay: Cv% < 12; sensitive: 0.03 ng/mL) and Bax (intra-assay: Cv% < 12; sensitive: 0.1 ng/mL) protein levels were measured using Rat ELISA Kit based on the manufacturer’s instruction (ZellBio GmbH, Ulm, Germany).
3.5. Statistical Analysis
Data were analyzed by SPSS version 20 using the Shapiro-Wilk and Levene tests for normal distribution of data and the assumption of the equality of variances, respectively. In addition, one-way analysis of variance (ANOVA) and LSD (least significant difference) post hoc test were employed for comparison between multiple groups. P ≤ 0.05 was considered the significance level.
4. Results
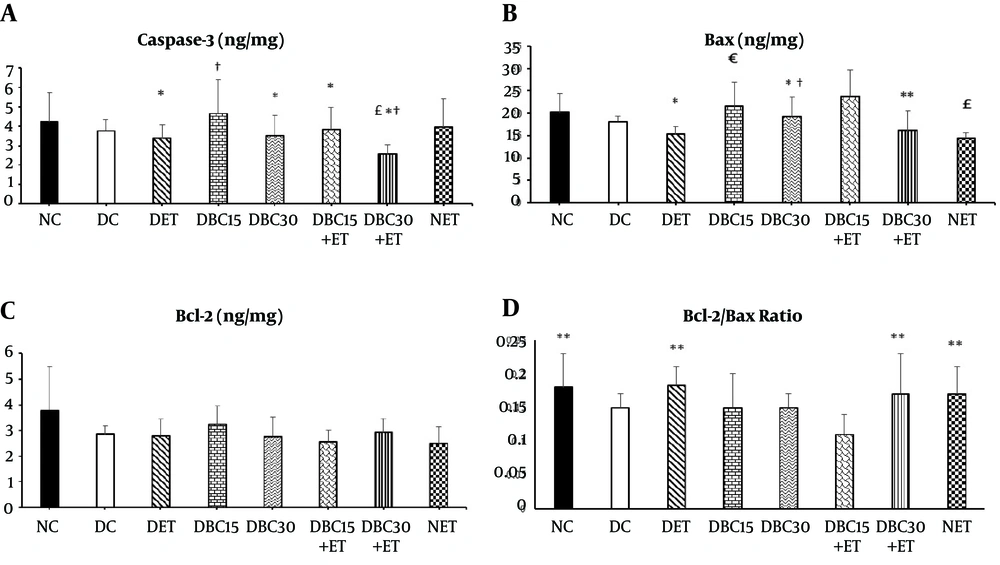
Based on the results, caspase-3 protein level demonstrated a reduction in DBC30+ET group (2.58 ± 0.48 ng/mg) compared to NC (4.23 ± 1.51 ng/mg), DC (3.78 ± 0.55 ng/mg), DBC15 (4.66 ± 1.73 ng/mg), and NET (3.96 ± 1.46 ng/mg) groups (P = 0.001, P = 0.02, P = 0.001, and P = 0.04, respectively). Caspase-3 protein level showed a decrease in DET (3.38 ± 0.67 ng/mg), DBC30 (3.52 ± 1.03 ng/mg), DBC15+ET (3.83 ± 1.15 ng/mg), and NET groups compared to DBC15 group (P = 0.0001, P = 0.01, P = 0.03, and P = 0.05, respectively). There was no significant different in the level of caspase-3 protein between NC and DC groups (P = 0.59) (Figure 1A).
The results of data analysis showed that Bax level significantly reduced in DET group (15.27 ± 1.79 ng/mg) compared to DBC15 (21.57 ± 5.41 ng/mg) and DBC30 (19.26 ± 4.41 ng/mg) groups (P = 0.02 and P = 0.001, respectively), and in DBC30+ET group (16.08 ± 4.45 ng/mg) compared to DBC15+ ET group (23.78 ± 5.92 ng/mg) (P = 0.001). On the other hand, Bax protein level significantly decreased in NET group (14.42 ± 1.20 ng/mg) than NC (20.19 ± 4.27 ng/mg), DBC15, and DBC30 groups (P = 0.03, P = 0.01, and P = 0.0001, respectively). There was no significant difference in the level of Bax protein between NC and DC groups (18.22 ± 1.14 ng/mg) (P = 0.49) (Figure 1B). The current study results also revealed no significant difference in Bcl-2 protein level after six weeks of ET and BC supplementation in renal tissue of the studied groups (P = 0.33) (Figure 1C).
Finally, the current study result showed an increase in the ratio of Bcl-2 to Bax in NC (0.18 ± 0.05) (P = 0.01), NET (0.17 ± 0.03) (P = 0.05), DET (0.19 ± 0.02) (P = 0.02), and DBC30+ET (0.17 ± 0.05) groups (P = 0.05) compared to DBC15+ET group (0.12 ± 0.02) (Figure 1D).
Effect of ET and different doses of BC on apoptotic indices in renal tissue (A to D). A, Caspase-3; B, Bax, C, Bcl-2, and D, Bcl-2 to Bax ratio. * Significant difference compared to DBC15 group; † significant difference compared to NET. £ significant difference compared to NC and DC; € significant difference compared to DET, ** significant difference compared to DBC15+ET. NC, normal control; DC, diabetes control; DET, diabetes exercise training; DBC15, diabetes berberine chloride 15 mg/kg; DBC30, diabetes berberine chloride 30 mg/kg; DBC15+ET, diabetes + BC15 + exercise training; DBC30+ET, diabetes + BC30 + exercise training; NET, normal exercise training.
5. Discussion
The current study examined the alteration of apoptotic indices in renal tissue of diabetic rats following six weeks of ET and in combination with different doses of BC. The current study results showed that the combination of ET with higher doses of BC reduced levels of pro-apoptotic proteins, while no significant change was observed in the level of anti-apoptotic protein (Bcl-2). However, the ratio of Bcl-2/Bax in NC, DET, DBC30+ET, and NET groups was higher than that of the DBC15+ET group.
Deficiency or inactivity of insulin due to a high level of glucose (hyperglycemia) can cause increased oxidative stress, associated with inflammation and apoptosis in the diabetic condition (13). The current study results demonstrated that ET combined with higher doses of BC may provide an effective protection and reducing the apoptotic activation during diabetic progression in STZ-induced diabetic male rats. While the Bcl-2 level as an anti-apoptotic protein had an insignificant difference in the renal tissue of ET or BC-treated diabetic rats. These observations were consistent with those of previous studies by Ramezani et al.; they demonstrated that combination of ET with higher doses of BC had more effectively decreases blood glucose, interleukin-6, and tumor necrosis factor-α than their individual effects (16). It is demonstrated that an increase in the ratio of Bax to Bcl-2 in damaged tissue can be considered as a reliable indicator of a cell to undergo apoptosis (18). The results of the present study showed that the ratio of Bcl-2 to Bax increased following ET and in combination with higher doses of BC. The apoptosis mechanism was investigated by ET, and it was suggested that the reduction of apoptosis pathway through ET can promote these benefits to the diabetic kidney. Although the results of the present study demonstrated an insignificant difference in apoptotic indices following a six-week ET in diabetic rats, the findings showed that regular ET prevented activation of apoptotic indices in normal groups. Aerobic exercise training provides protective action on the diabetic kidney, but its molecular mechanism is not elucidated clearly. However, there is evidence for the improvement of the redox state (19). Amaral et al., demonstrated that a four-week aerobic exercise training through reduction of renal inflammation markers and oxidative stress promotes protective effects in the kidney of STZ-induced diabetic female rats (19). The components of glucose metabolism, ROS, and antioxidant system were not investigated in the current research, which is considered as a limitation of the study. Amaral et al., reported several changes in renal cortex of STZ-induced diabetic female rats including enhancement of metabolic homeostasis and renal function, a reduction in kidney morphology modifications, proteinuria decrease, as well as down regulation in the expression of extracellular matrix (ECM) proteins after moderate-intensity ET (20). Kim et al., showed an increase of Bcl-2 expression in STZ-induced diabetic rats after a six-week treadmill exercise (21); these results were inconsistent with those of the current study. The components of caspase-independent apoptotic pathways (e.g., apoptosis-inducing factor) were not investigated in the current study; therefore, further studies are required to determine the sensitivity of this pathway to ET combined with BC supplementation through non-caspase-dependent markers.
In addition, it was demonstrated that 30 mg/kg of BC had a greater effect on the inflammatory blood parameters compared to its 15 mg/kg in the STZ-diabetic rats (16). Another study also demonstrated that chronic BC treatment had a beneficial effect on synaptic dysfunction and anti-apoptotic properties in STZ-diabetic rats (22). Chandirasegaran et al., investigated the efficacy of different concentrations of BC (25, 50, and 100 mg/kg) on hyperglycemia in STZ-induced diabetic rats and reported that 50 mg/kg of BC would reduce blood glucose, glycation of hemoglobin, and levels of renal markers (12). Based on the studies, the molecular mechanisms undertaking BC against diabetes mellitus include lipid and glucose metabolism, and antioxidant and anti-inflammatory activities that have therapeutic activities against diabetes mellitus and insulin resistance (23-25). A pilot study indicated that BC treatment was safe in patients with type 2 diabetes and it had beneficial effects on lipid metabolism (24). Enhancement of glucose metabolism by the BC treatment may be due to glycolysis stimulation related to mitochondrial inhibition of glucose oxidation (26). According to evidence, berberine effectively inhibits the activity of disaccharidases and decreases glucose transportation across the intestinal epithelium (24). Hence, the anti-apoptotic and antioxidant properties of BC may be credited for all of the above effects. According to the best of authors’ knowledge, there were no studies on the effect of ET combined with BC supplementation on apoptotic indices in patients with diabetes. Thus, new results of ET combined with higher doses of BC supplementation elucidated inhibition of apoptosis in renal tissue of STZ-induced diabetic rats. Further studies are required to obtain more conclusive results.
5.1. Conclusions
In conclusion, treatment with ET combined with 30 mg/kg of BC notably inhibited the mitochondrial-mediated caspase-dependent apoptotic pathway in the kidneys of diabetic rats. The current study findings showed that supplementation with higher doses of BC combined with ET had the potential to protect against apoptosis than the lower doses of BC and gives hope for the curative and prophylactic drug to treat diabetes mellitus.