1. Background
Dental caries is among the most important infectious diseases of the oral cavity (1). It first involves the enamel and is characterized by the loss of minerals meanwhile, the superficial tooth structure appears to be intact. Increased porosities in the underlying tissue lead to the formation of a white appearance. This lesion is referred to as a white spot lesion (WSL) (2).
Remineralization refers to the replacement of the minerals lost during the demineralization process (3). The efficacy of local treatments for induction of remineralization of incipient carious lesions has been previously confirmed. This process stops the progression of caries and reverses its course. Therefore, early detection of WSLs and their treatment with remineralizing agents is of high significance (4).
The presence of orthodontic brackets, dental crowding and malocclusion cause dental plaque retention and complicate adequate oral hygiene practice, leading to plaque accumulation on the tooth surface (5). WSLs are commonly observed on the buccal and labial surfaces of the teeth around orthodontic brackets and they are often covered with dental plaque. The WSLs, as a common complication of orthodontic treatment, compromise dental esthetics following orthodontic treatment and bracket removal (6, 7).
Management of WSLs following orthodontic bracket removal is challenging (5) and they are often resistant to remineralization (8). On the other hand, they negatively affect dental esthetics. (5). Thus it is important to detect WSLs around orthodontic brackets as early as possible because lesions detected early may be remineralized and eliminate the need for further complex treatments (9).
The role of fluoride in the prevention of dental caries has been well-recognized. The fluoride ions replace the hydroxyl ions in hydroxyapatite (HA) crystals and result in the formation of more stable fluorapatite crystals, which are more resistant to acid attacks. This effect is specifically prominent when the pH of the environment around the enamel drops (10). Evidence shows that patients who use fluoride during their orthodontic treatment have fewer WSLs by 44.3% compared to controls (11). Despite all the advantages of fluoride, its effect on carious lesions of the pits and fissures is limited. Also, high doses of fluoride are associated with the risk of fluorosis. Thus attempts are ongoing to find an alternative remineralizing agent (12).
On the other hand, HA is a rich source of calcium and phosphate, which is highly efficient for remineralization of demineralized enamel. In fact, all mineral structures of the human body are made of calcium and phosphate with lower amounts of calcium carbonate and calcium sulfate. Synthetic HA forms a new layer of artificial enamel on the tooth surface to protect it meanwhile, it further strengthens the existing layer by chemically altering its structure and forming fluorapatite (13).
Advances in nanotechnology in different fields of science led to the production of nano-hydroxyapatite (nHA) particles, which have a high affinity for proteins and parts of dental plaque and bacteria. This affinity is related to the size of nanoparticles and their increased surface area for bonding to proteins (13). The nHA particles are hydrophilic and can moisturize the surface. When applied to the tooth surface, they produce a strong, thin layer on the enamel surface that bonds to the tooth structure. The nHA particles cause enamel remineralization. Moreover, nHA serves as a filler and fills the porosities on the enamel surface due to the very small size of particles (13, 14).
A scanning electron microscope (SEM) is commonly used for microscopic studies. It provides magnified images and can also be used for chemical analysis. Its mechanism of action is based on the interaction of electron beams with the object. The radiated beams can be used for assessments. SEM uses electron beams instead of light. Since the electron wavelength can be very short, very high magnification can be obtained by the use of an SEM (up to one million times in some SEMs) (15).
To date, many studies have evaluated the application of nHA in different forms such as toothpaste, paste, and gel for remineralization of incipient carious lesions. However, studies on the efficacy of different concentrations of nHA mouthwashes for remineralization of WSLs around orthodontic brackets are limited (16-18).
2. Objectives
Therefore, this study aimed to assess the mineral content of WSLs around orthodontic brackets after remineralization with 1%, 5%, and 10% nHA mouthwashes in comparison to fluoride gel using an SEM.
3. Methods
This in vitro experimental study evaluated 50 sound premolars extracted for orthodontic treatment (ethical approval code of IR.SEMUMS.REC1396.186). The teeth were caries-free and had no fracture or chipping. The enamel surface was polished with pumice paste, rinsed with deionized water and air-dried. The mineral content of the teeth was measured by the SEM/EDX analysis before the intervention and recorded as the baseline value. The applied system presents one EDX detector used for qualitative and quantitative microanalysis. The X-flash-6® detector is the third generation of detectors that does not require liquid nitrogen cooling and is about 10 times faster than the conventional Si (Li) detectors. The X-rays have to pass the radiation entrance window of the detector before entering the active volume of the detector. Normalized high-resolution spectra of the main elements’ concentration in weight percent were performed and later calculated by an EDX using the backscattered electron collector connected to a scanning electron microscope (TESCAN Model VEGA II\XMU, Brno, Czech Republic) operating at 30 kV and working distance of 20 mm.
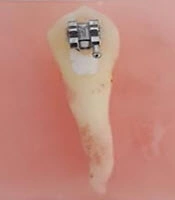
For standardization and decreasing the risk of enamel exposure to acid, the tooth surface was coated with wax. The wax was cut from the buccal cusp tip by 2 mm gingivally and at the center of the tooth in a mesiodistal direction equal to the size of a bracket. The enamel surface of the created window was then etched with 35% phosphoric acid (Reliance, Itasca, IL, USA) for 30 seconds (Figure 1). After observing a chalky white appearance, the surface was completely rinsed and dried. Single Bond (3M ESPE, St. Paul, MN, USA) was applied to the area with a micro-brush and light-cured for 25 seconds using a light-curing unit (3M/Unitek). Composite (3M ESPE, St. Paul, MN, USA) was applied on the back of each bracket using a composite instrument and the bracket was then placed on the tooth surface. The excess composite was removed by an explorer and light-curing was repeated for another 25 seconds. Next, the wax was removed from the tooth surface. The tooth surface was then sealed with nail varnish (which is an acid-resistant enamel) except for a rectangular window measuring 3 × 2 mm cervical to the bracket, (Figure 2) which had the highest risk of development of WSLs due to difficult access for oral hygiene maintenance. This area was subjected to artificial demineralization. The teeth were immersed in a cariogenic solution (Polymer Research Center, Iran) for 9 days. The solution was refreshed every 48 hours. The cariogenic solution had a pH of 4 and contained 0.5 g yeast extract, 1 g glucose, and 2 g sucrose. Several studies show that such a procedure using artificial cariogenic solutions can simulate WSLs as they form in the oral environment (19, 20). The formed biofilm was wiped off by a wet gauze and the nail varnish was removed with a scalpel. The teeth were copiously rinsed with deionized water. The WSLs were induced as such (Figure 3).
The teeth were randomly divided into 5 groups (n = 10) for immersion in artificial saliva (control group), 1.23% fluoride gel (group 2), 1% nHA mouthwash (Pardis Pajouhesh Fanavaran, Yazd) (group 3), 5% nHA mouthwash (group 4), and 10% nHA mouthwash (group 5). The teeth were exposed to fluoride gel/nHA mouthwashes twice with a one-week interval. The gel or mouthwash was wiped off from the tooth surface after 24 hours using sterile gauze and the teeth were rinsed with 15 cc of deionized water. After the application of fluoride gel/mouthwashes, the teeth were stored in artificial saliva for one week. After 2 weeks, the mineral content was evaluated once again with the mentioned method. Data were analyzed using the Shapiro-Wilk test, one-way ANOVA, and Tukey’s post hoc test via SPSS version 18.0 (SPSS Inc., IL, USA). The level of significance was set at 5%.
4. Results
4.1. Calcium Content
Table 1 presents the mean and standard deviation of calcium content after the intervention compared to baseline. Prior to demineralization (at baseline), the mean calcium content was not significantly different among the five groups (P = 0.991). However, the five groups were significantly different in terms of it after the intervention (P < 0.001) such that the calcium content of the control group was significantly lower than that of the fluoride gel and 1%, 5%, and 10% nHA mouthwashes (P < 0.001, P < 0.001, P < 0.001, and P < 0.002, respectively). Also, the calcium content of the fluoride gel group was significantly higher than that of the 5% nHA (P < 0.001) and 1% nHA (P < 0.001) groups. The calcium content of teeth in the 10% nHA group was significantly higher than that of teeth subjected to other concentrations of nHA mouthwash (P < 0.001) but had no significant difference with the calcium content of the fluoride gel group (P = 0.997). The calcium content of the 1% nHA and 5% nHA groups showed significant differences compared with that of other groups but the difference between the two was not significant (P = 0.062).
| Group | Baseline (Before Demineralization) | After the Intervention | ||
|---|---|---|---|---|
| Mean ± SD | P Value | Mean ± SD | P Value | |
| Control | 37.49 ± 0.09 | 0.991 | 29.01 ± 0.63 | < 0.001 |
| Fluoride gel | 37.47 ± 0.10 | 33.04 ± 0.84 | ||
| 10% nHA | 37.48 ± 0.09 | 33.15 ± 0.71 | ||
| 5% nHA | 37.47 ± 0.09 | 31.16 ± 0.67 | ||
| 1% nHA | 37.48 ± 0.09 | 30.28 ± 0.70 | ||
4.2. Phosphorus Content
Table 2 presents the mean and standard deviation of phosphorus content after the intervention compared to baseline. Prior to demineralization (at baseline), the mean phosphorus content was not significantly different among the five groups (P = 0.998). However, the five groups were significantly different in terms of it after the intervention (P < 0.001) such that the phosphorus content of the 10% nHA (P < 0.001), 5% nHA (P < 0.001), and fluoride gel groups was higher than compared with the control group but the 1% nHA group showed no significant difference compared with the control group (P > 0.05). Also, the phosphorus content of 5% and 10% nHA and fluoride gel groups was significantly higher than compared to the 1% nHA group (P < 0.001). The phosphorus content of teeth in the 10% nHA group was higher than the 5% nHA (P = 0.018) and the other groups (P < 0.001). The phosphorus content of the 5% nHA group had no significant difference compared with the fluoride gel group (P = 0.437), but was significantly lower than that of teeth in 10% nHA group (P = 0.018) and was significantly higher than that of teeth in the 1% nHA and control groups (P < 0.001).
| Group | Baseline (Before Demineralization) | After the Intervention | ||
|---|---|---|---|---|
| Mean ± SD | P Value | Mean ± SD | P Value | |
| Control | 18.12 ± 0.51 | 0.998 | 14.07 ± 0.44 | < 0.001 |
| Fluoride gel | 18.18 ± 0.56 | 14.75 ± 0.52 | ||
| 10% nHA | 18.14 ± 0.59 | 16.05 ± 0.55 | ||
| 5% nHA | 18.15 ± 0.61 | 15.20 ± 0.89 | ||
| 1% nHA | 18.19 ± 0.53 | 14.28 ± 0.42 | ||
4.3. Fluoride Content
Table 3 presents the mean and standard deviation of fluoride content after the intervention compared to baseline. Prior to demineralization (at baseline), the mean fluoride content was not significantly different among the five groups (P = 0.458). However, the five groups were significantly different in terms of it after the intervention such that the fluoride content of the fluoride gel group was significantly higher than that of the other groups (all Ps < 0.001). Also, the fluoride content of the control group was less than that of the 10% (P < 0.001), 5% (P < 0.001) and 1% (P < 0.001) nHA groups. The fluoride content of teeth in the 10% nHA group was significantly higher than that of the 5% (P < 0.001) and 1% (P < 0.001) nHA groups.
| Group | Baseline (Before Demineralization) | After the Intervention | ||
|---|---|---|---|---|
| Mean ± SD | P Value | Mean ± SD | P Value | |
| Control | 0.027 ± 0.005 | 0.458 | 0.014 ± 0.005 | < 0.001 |
| Fluoride gel | 0.026 ± 0.005 | 0.078 ± 0.006 | ||
| 10% nHA | 0.026 ± 0.005 | 0.075 ± 0.008 | ||
| 5% nHA | 0.025 ± 0.005 | 0.037 ± 0.005 | ||
| 1% nHA | 0.023 ± 0.005 | 0.037 ± 0.005 | ||
5. Discussion
This study aimed to assess the changes in calcium, phosphorus, and fluoride content after the use of fluoride gel and nHA mouthwash with different concentrations for remineralization of WSLs in teeth with orthodontic brackets to assess their efficacy for this purpose. The results showed that calcium intake was maximum in teeth exposed to fluoride gel and 10% nHA mouthwash. Maximum phosphorus uptake was noted in teeth exposed to 10% nHA followed by fluoride gel and 5% nHA; however, the latter two were not significantly different. The fluoride uptake was the highest in teeth in the fluoride gel group with significant differences with other groups followed by 10% in the nHA group.
One major goal of preventive dentistry is to stop the progression of incipient carious lesions and induce their remineralization. This is done to prevent the extension of carious lesions and preserve the tooth structure (19).
Application of nHA mouthwash is a suggested technique for the prevention of dental caries. Nano-HA is a calcium phosphate compound with a structure resembling the mineralized part of dentin and enamel. It can remineralize the incipient carious lesions (20). Several studies have evaluated the remineralizing efficacy of nHA for demineralized enamel and dentin lesions (21-23).
Kim et al. (24) in 2007, evaluated the degree of remineralization following the use of 0%, 1%, 5%, and 10% nHA in distilled water and the same concentrations of nHA in 0.05% sodium fluoride. They evaluated 48 demineralized enamel samples using confocal laser scanning microscopy and concluded that nHA and fluoride have a synergistic effect. In their study, similar to ours, the rate of remineralization was increased by an increment in the concentration of nHA (25). However, Kim et al. evaluated the synergistic effects of nHA and fluoride, and nHA and distilled water. However, our study compared the effects of pure nHA and pure fluoride gel.
Najibfard et al. (26), in an in situ study in 2011, evaluated the effect of 1100 ppm fluoride, 10% nHA, and 5% nHA toothpaste for remineralization of primary enamel lesions. They concluded that all three kinds of toothpaste enhanced the process of remineralization and there was no significant difference among them (27). In the present study, the remineralizing capacity of nHA significantly increased by an increase in concentration. This difference in the results of the two studies may be due to the application of different forms of nHA since mouthwash was used in our study while Najibfard et al. applied toothpaste. Similarly, Daas et al. in 2018, evaluated and compared the efficacy of nHA paste and fluoride varnish for the protection of dental surfaces against demineralization. They selected 45 dental samples artificially induced carious lesions in them and evaluated them before and after the exposure to nHA paste and fluoride varnish using an SEM. They found no significant difference in resistance to demineralization between the two materials. The difference between their findings and ours may be attributed to the application of different forms of fluoride and nHA as well as different concentrations (16).
Similar to the current study, Bajaj et al. (28), in 2016 used a polarized light microscope to assess the remineralization of WSLs on primary molars following the application of tricalcium phosphate, nHA and casein phosphopeptide amorphous calcium phosphate pastes. They concluded that nHA paste had a higher potential to induce remineralization than the other two compounds (24).
Many studies have evaluated the remineralizing effects of fluoride in different forms and similar to the current study, confirmed its efficacy for enamel remineralization (26, 28, 29). However, previous studies have used different forms of fluoride such as varnish, paste, gel and mouthwash alone or in combination with other factors. Moreover, the methods employed for the assessment of remineralization have been variable, including measurement of microhardness, atomic force microscopy, polarized light microscopy, and SEM. Therefore, differences in the results of studies may be attributed to these factors. However, studies on remineralization of incipient caries around orthodontic brackets are limited and the majority of the available ones have evaluated the effect of fluoride alone or its synergistic effect with other remineralizing compounds (20, 30-32). Finally, the authors suggest that more studies are required to compare various forms of remineralizing agents in the clinical status.
5.1. Limitations
The present study compares the remineralization effects of various concentrations of n-HA in the form of mouthwash and fluoride gel while several remineralizing agents exist that should be tested and studied in order to find the best. Further studies are also recommended to evaluate in vivo situations.
5.2. Conclusions
Considering the results of this study, it may be concluded that nHA mouthwash in different concentrations can effectively remineralize the WSLs around orthodontic brackets and can be used as an alternative to fluoride for this purpose.
.jpg)


