1. Background
Tuberculosis (TB) is one of the major public health problems whose burden remains more challenging for health care experts to address (1). Recent estimates show that nearly 1.3 million deaths occurred due to TB during 2017 which is excluding an additional 300000 deaths accounted by TB in the HIV positive population, which makes TB a leading cause of mortality from an infectious disease (2). The World Health Organization (WHO) Report for Global TB Report 2018 reveals that nearly 10 million people acquired TB in 2017 (2). Though the reports suggest a mild decline in the incidence of TB (1), multi-drug resistant tuberculosis (MDR-TB) remains as a significant threat to the accelerated control efforts globally (3). Across the world 3.5% of the new cases and 18% of the previously diagnosed cases have been reported to have MDR-TB, with India remaining the country with highest percentage (24%) of MDR-TB reported (2). In a high burden country like India, the latent TB infection is more prominent than reactive TB cases (4). Further TB infections impair the lungs which makes the patient vulnerable to environmental pathogens and leaves them in an immune-compromised state (5, 6). Oxidative stress is regarded as one of the major rate-limiting steps in the progression of TB (7-9). The enhanced generation of reactive oxygen species (ROS) generated as a host defensive mechanism against mycobacterium increases tissue injury and inflammation which can lead to immunosupression (7). Using a suitable antioxidant therapy is suggested as a beneficial approach for faster recovery (7, 10-12).
Ozone, a molecule discovered in the mid-19th century, has shown to optimize anti-oxidant systems and metabolic functions of the body. The antioxidant property of ozone is attributed to its strong oxidizing property (13, 14). Thus it can restore the cellular redox balance which is impaired in infectious diseases like TB (14, 15). In 1991 Priimak et al. demonstrated that a mixture of ozone and oxygen can reduce the reproductive capacity of Mycobacterium tuberculosis which is suggestive that the use of ozone and oxygen mix can be promising intervention in the management of TB (16). Several in vivo and in vitro experiments in animals and humans advocate dissolved ozone to enhance the effect of drugs in MDR-TB (17, 18).
2. Objectives
We investigated the role of ozone therapy in boosting the anti-oxidant status of MDR-TB patients.
3. Methods
The Institutional Ethics committee of ozone forum of India approved the study protocol. This study included seven patients diagnosed with MDR-TB who were under AKT-4 (Anti Koch’s treatments) in the critical care department of a government tertiary care setting in Mumbai, India, exclusively dedicated to treating TB cases. Written consent was taken from the patients who were included in the study after explaining the role of ozone therapy and its safety considerations. All the patients were given 21 sessions of rectal ozone insufflations at a rate of 3 sessions per week. Additionally, sterile ozonized water wash was given to those patients who had emphysema surgeries.
Rectal insufflation is a method by which an ozone-oxygen mixture is administered through the rectum. The patients were asked to lie in the left lateral position to prevent any gastric discomfort and release of flatus. The interventional dose was 500 mL of ozone-oxygen mixture at 35 mcg/mL concentration; introduced into the rectum using a No. 10 infant feeding tube, up to 4” deep; after lubricating the tip of the tube with ozonated oil. Then the rectal bag was filled with ozone oxygen mixture is attached to the infant feeding tube and the ozone is dispensed slowly into the rectum by squeezing the bag gently and then rolling it circularly. The flow rate was set at 120 mL per minute to achieve the desired concentration. The procedure should be very slow and gentle to avoid any back pressure from the rectum to the bag. The whole procedure should take 3 - 4 minutes. The patient has to lie down on the back for 2 - 3 minutes for better absorption. The ozone/oxygen mixture was generated from an ozone generator for medical use (O3-Ozonics generator, Ozone Forum of India), which is automated and standardized for time, volume and concentration (19).
Blood serum and plasma samples were collected from the patients at the baseline and at the end of the 21st session to assess the anti-oxidant status of superoxide dismutase (SOD) and glutathione reductase (GR). The SODs form the first line of defense against the injuries mediated by reactive oxygen species (ROS) (20). Being an important anti-oxidant defense agent, SOD catalyzes the reaction by which superoxide anion free radical (O2-) dismutase into molecular oxygen and hydrogen peroxide (H2O2). This will decrease O2- level in the cells which is a rate-limiting step in preventing oxidative stress injury (21). In addition, GR is another potent antioxidant agent that protects the cells from oxidative damage. This is achieved by a rise in the level of reduced glutathione which neutralizes the O2- levels (22). Thus an increase in these antioxidants’ levels favors better prognosis in high oxidative stress states like TB.
Serum Superoxide dismutase was analyzed by a commercially available kit from Cayman Chemicals, USA (Cat no 706002). This assay utilized a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. The SOD activity was expressed as U/mL.
Plasma Glutathione reductase assay was also performed using Cayman chemicals, USA (Cat no 703202) kits. This assay kit measured GR activity by measuring the rate of NADPH oxidation. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm and is directly proportional to the GR activity in the sample. GR activity was expressed as nmol/min/mL.
4. Results
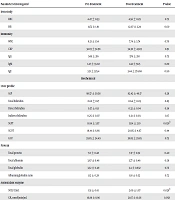
Statistical Package for the Social Sciences (SPSS) 16.0 was used for the data analysis. A paired sample t-test was used for comparison between pre-treatment and post-treatment changes. Out of the seven participants, there were two females and five males with a mean age of 31.86 ± 9.4. Statistically, a significant rise was observed in SOD (P = 0.028), but not in GR (P = 0.058). No other biochemical profiles have shown any statistically significant change except for reduction in serum glutamic-pyruvic transaminase (P = 0.026). The results are tabulated in Table 1.
| Parameters Investigated | Pre-Treatment | Post-Treatment | P Value |
|---|---|---|---|
| Iron study | |||
| RBC | 4.47 ± 0.53 | 4.56 ± 0.29 | 0.72 |
| HB | 11.72 ± 1.91 | 12.07 ± 2.24 | 0.60 |
| Immunity | |||
| WBC | 8.21 ± 1.54 | 7.74 ± 3.74 | 0.70 |
| CRP | 32.03 ± 32.98 | 34.30 ± 43.83 | 0.91 |
| IgG | 549 ± 216 | 576 ± 210 | 0.75 |
| IgM | 1.47 ± 33.02 | 1.42 ± 58.5 | 0.80 |
| IgE | 3.31 ± 325.4 | 3.44 ± 374.68 | 0.90 |
| Biochemical | |||
| Liver profile | |||
| ALP | 98.57 ± 30.58 | 82.42 ± 46.17 | 0.28 |
| Total bilirubin | 0.62 ± 0.17 | 0.64 ± 0.05 | 0.85 |
| Direct bilirubin | 0.37 ± 0.11 | 0.32 ± 0.04 | 0.36 |
| Indirect bilirubin | 0.25 ± 0.07 | 0.31 ± 0.03 | 0.17 |
| SGPT | 18.14 ± 3.97 | 13.14 ± 2.19 | 0.026b |
| SGOT | 19.14 ± 6.66 | 20.85 ± 4.67 | 0.44 |
| GGT | 39.85 ± 24.49 | 38.85 ± 29.85 | 0.75 |
| Protein | |||
| Total protein | 7.0 ± 0.48 | 7.17 ± 0.52 | 0.40 |
| Total albumin | 3.67 ± 0.48 | 3.77 ± 0.46 | 0.59 |
| Total globulin | 3.32 ± 0.49 | 3.4 ± 0.832 | 0.76 |
| Albumin/globulin ratio | 1.12 ± 0.28 | 1.18 ± 0.52 | 0.72 |
| Antioxidant enzyme | |||
| SOD, U/mL | 1.51 ± 0.61 | 2.09 ± 1.07 | 0.028b |
| GR, nmol/min/mL | 16.88 ± 8.96 | 21.67 ± 10.36 | 0.058 |
Pre and Post Evaluation of Ozone Therapy in MDR-TBa
5. Discussion
The present study demonstrates the role of ozone therapy in improving the antioxidant activity in immune-compromised conditions such as tuberculosis. The results are identical to the previous in vitro and vivo studies conducted which demonstrated a reduction in oxidative stress and enhanced antioxidant defense mechanisms post ozone interventions (14-18). Furthermore, ozone is reckoned as a strong anti-microbial agent that can destroy the cellular cavities as well as reduce the reproductive capacity of the microbial cells (16, 23). Ozone improves the cellular redox balance and increases the oxygen uptake by the cells (13, 14). This action supplements the innate immunity of the body (24). Basic science research also explains the beneficial effects of ozone therapy in destroying microorganisms including bacteria and viruses (24). MDR-TB is considered emergent problem despite the improvement of public health and medical services (25), and ozone therapy can be taken in to account as a vital complementary therapy that can enhance the potency of existing care.
Published literature suggests that the possible mechanism by which ozone therapy induces beneficial effects is by upregulating cellular antioxidant enzymes and induction of heme-oxygenase-1 (HO-1) and heat-shock proteins (HSP70) (26). These free antioxidants protect the cells from oxidation and inflammation, thereby reverse the chronic oxidative stress (27). Furthermore, it enhances the release of growth factors.
Another possible mechanism by which ozone therapy acts is by influencing the immunoglobulins (Ig). Studies have reported an increase in IgG, IgM, and IgA which is attributed to the prevention of recurrence in infections. Immunoglobulins play a large role in the humoral response of adaptive immunity, which helps in maintaining immune system homeostasis (28-31). Our study has also shown moderate increase in IgG and IgM levels. However, these changes were not statistically significant. The smaller sample size may be a reason for the statistical insignificance.
Besides the improvement in antioxidant markers, the participants expressed positive subjective changes in their mental status. However, this is based on the personal experiences shared by the patients with the investigators. No standardized scales were used to validate these psychological changes, which remains one of the drawbacks of this study. The psychological well-being post ozone therapy is attributed to the effect of ozone therapy on the neuroendocrine system (32).
Though the present study exhibits promising results, the smaller sample size is a limitation which will limit us from generalizing this result to a larger population. This further affects the effect size of the parameters under study as we have observed in GR. Lack of potential control group remains another weakness of this study. Large randomized control trials are warranted to use ozone effectively in MDR-TB.
5.1. Conclusions
In light of the current results, it is evident that ozone can be a promising medium in tackling the oxidative stress and to enhance the positive mental attitude in ailing MDR-TB patients. Medical ozone therapy can, therefore, be considered as an adjuvant in managing multidrug-resistant TB (MDR-TB).