1. Background
Prostate cancer (PrCa) is the most dangerous and common cancer in men. The main problem arising from prostate cancer is its tendency to metastasis. Metastasis occurs to infiltrate surrounding tissue, which leads to local invasion, extravasation and distal migration from the primary site, followed by endothelial-dependent, transmigration and the site-specific establishment of metastases at the secondary site (1).
Exercise and cancer affect angiogenesis, angiogenesis can be viewed as a phenomenon linking exercise, and cancer (2). It has been suggested that exercise training and plant-derived phytochemicals could use as a potential supportive therapy to reduce the progression of cancer (3). However, exercise training in cancer patients has always been cautious because it could increase metastasis and angiogenesis factors (4). On the other hand, research has demonstrated that moderate aerobic exercise training compared to high-intensity training was more effective to suppress angiogenesis markers in tumor tissue (5).
Matrix metalloproteinases (MMPs) are a series of zinc-dependent endo-peptides, which play vital roles in cancer cell invasion, tumor growth, and metastasis (6). Indeed, MMPs can degrade many extracellular matrix (ECM) proteins without a change in the formation of tumor blood vessels (7). Many MMPs such as MMP-2/-9 can be protective in cancer and their up-regulation may be involved in processes aimed at eliminating abnormal tumor cells. It is demonstrated that endothelial cells are capable of differentially expressing and activating MMPs (8). It has been investigated that vascular endothelial cell growth factor (VEGF) as a critical angiogenic factor for angiogenic switch is required that delivered into the tumor microenvironment by the MMP-9 and release from tumor matrix by MMPs (9).
One of the factors that may reduce the risk of PrCa is the diet that it is a modifiable factor that may play an important role in the prevention of several types of cancer (10). There is convincing evidence from previous studies that green tea has chemo-preventive effects (11). Epigallocatechin-3-gallate (EGCG), the green tea component is believed to be a major contributor to these effects (12). EGCG has been demonstrated to inhibit cell growth via apoptotic cell death in human PrCa cells (13), EGCG not only reduces secreted VEGF in cancer cell but also inhibits tumor-associated macrophages (TAMs) in endometrial cancer (14). Inhibition of tumor invasion (15) and angiogenesis (16) as two critical steps for the growth and metastasis of solid tumors has been attributed to the chemopreventive activity of green tea extract (17).
2. Objectives
Exercise training in cancer patients has always been cautious because it could increase metastasis and angiogenesis factors (4). It is stated that endurance training with moderate intensity, in particular, causes angiogenesis (3); therefore, it is concerned that endurance training though the angiogenesis can lead to metastasis in tumor tissue in patients with cancer. On the other hand, exercise training and plant-derived phytochemicals (green tea) could use as a potential supportive therapy to reduce the progression of cancer (3). However, the interaction of changes in angiogenesis and metastasis with regular aerobic training and green tea extract is not well defined. In the present study, we evaluated the effects of eight weeks of aerobic training and green tea extract consumption on protein levels of MMP-2, MMP-9, and VEGF in both experimental groups; with and without prostate cancer Wistar rats.
3. Methods
3.1. Animals and Experimental Design
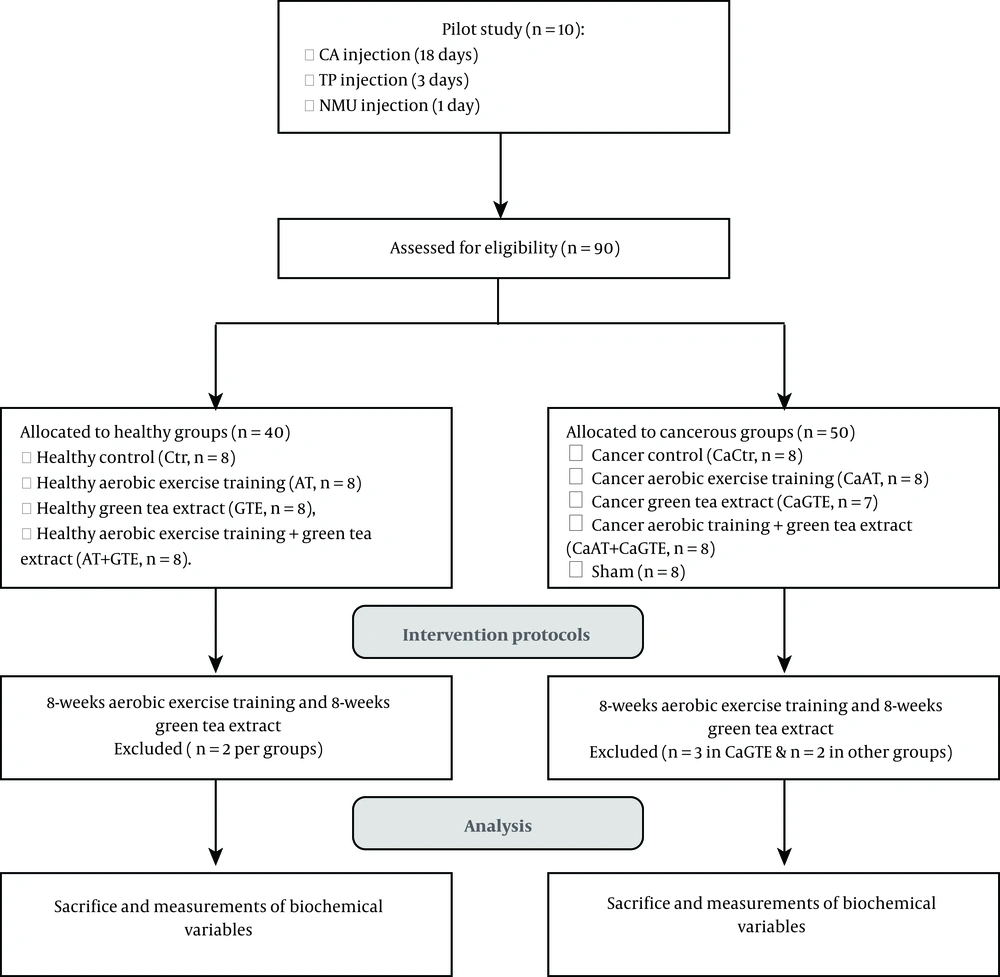
All procedures were approved by the Ethics Research Committee of Kurdistan University of Medical Sciences, Iran, IR.MUK.REC.1396.184. Ninety (8 weeks-old, bodyweight 250 ± 20 g) Wistar rats (obtained from Pasteur Institute of Iran, Karaj, Iran) were used. The rats were housed, accredited temperature (22 ± 2°C), light controlled facilities (12:12 hours), and provided standard rat chow and water ad libitum. The animals were randomly assigned to two healthy and cancer groups and then the healthy group was divided into four subgroups, including control (Ctr, n = 8), healthy aerobic training (AT, n = 8), healthy green tea extract (GTE, n = 8), healthy aerobic training + green tea extract (AT + GTE, n = 8). The healthy rats were kept for five months until cancer protocol was performed in the cancer groups. Then the study simultaneously began after inducing cancer in rats; afterward, the cancer group was divided into five subgroups, including cancer control (CaCtr, n = 8), cancer AT (CaAT, n = 8), cancer GTE (CaGTE, n = 7), cancer AT + GTE (CaAT + GTE, n = 8) and sham (n = 8) groups. It should be noted that the first of all the number of cancer groups was 10 rats, but during the intervention, two rats were lost from each group except the GTE group that three rats died and others remained as mentioned. The rats used in the present study are a subset of the cohort in our recently published paper (18). The flowchart and timeline of the study are summarized in Figure 1.
It should be noted that initial weighting of rats was measured after the onset of cancer and final weighing was performed before anesthetized with using a Sartorius ENTRIS 3202-1S with precision balance offers 3200 grams weighing capacity with readability to 0.01 grams.
3.2. Tumor Induction
In the cancer groups, tumor induction was carried out by a modified method described in the previous studies (Ashe Stevens, Detroit, MI, Bosland and Prinsen) (19). For this purpose, cancer group rats received daily cyproterone acetate (Sigma-Aldrich, St Louis, MO, USA) (CA: 50 mg/kg body weight in sesame oil) by intraperitoneal injection for 18 consecutive days. Rats, one day after the final dose of cyproterone acetate, received daily subcutaneous injections of testosterone propionate (TP) (Sigma-Aldrich, St Louis, Mo, USA) (100 mg/kg body weight in sesame oil) for three days. Rats received a single intravenous injection of N-nitroso-N-methylurea (NMU: 50 mg/kg body weight in sesame oil) on the day after of testosterone propionate administration. The NMU was first wetted with 3% acetic acid and then diluted with normal saline to prepare a final concentration of 10 mg/mL with pH = 5.5 for injection (17, 19). To order control of stress due to injection we used the sham group.
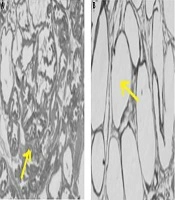
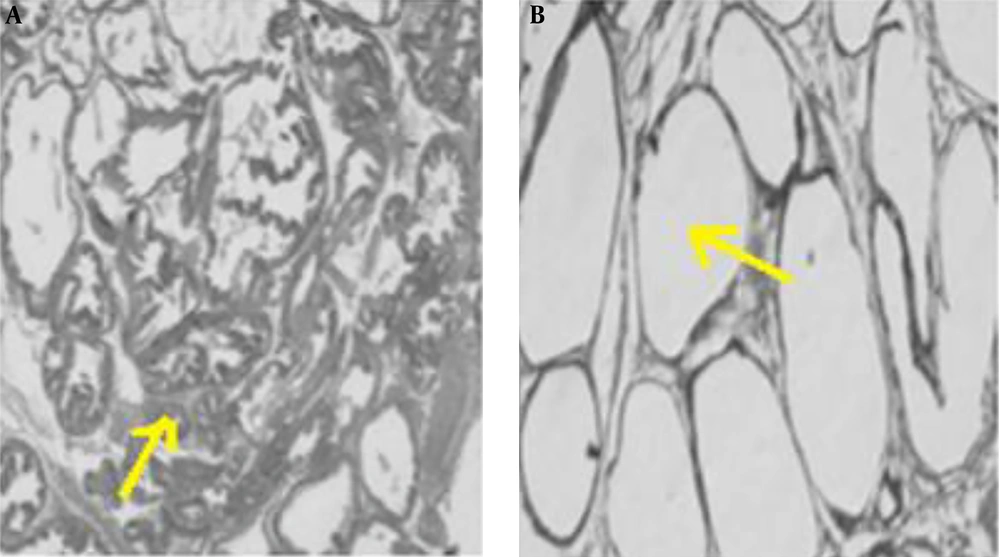
To ensure that the rats are cancer and, before conducting the research study, a pilot study was conducted to induce prostate cancer, and after ensuring the induce cancer model (histological examination of prostate was used for diagnosis, unwell-differentiated epithelium with local invasion was observed for this purpose), the original research was started. In the pilot study, fresh tissue of the prostate was fixed in 10% v/v phosphate-buffered formalin (pH = 7.2) and embedded in paraffin. Sections (5 µm) were prepared onto slides and stained with Harris hematoxylin, followed by 1% eosin (H&E). Then the slides were dehydrated in 95% and 100% ethanol, cleared in xylene and mounted with Permount. The results are shown in Figure 2 and histological images represent the method used to induce prostate cancer in this study after 5 months (Figure 2A).
3.3. Aerobic Training Intervention
The aerobic training protocol was included training on a treadmill for 5 days a week with 45 minutes in three 15-minute work with two minutes of rest between each period of work as previously described (18). Aerobic training intensity in order to achieve the target speed, in every week, 1 m/min was added to the treadmill speed. The intensity of the given exercise training is equal to 60% of maximal aerobic capacity (low-to-moderate exercise intensity domain) for animals with prostate cancer. Aerobic training began with 30% VO2max intensity in the first of four weeks and reached 40% VO2max in the eighth week in healthy groups (18).
3.4. Preparation and Consumption of Green Tea Extract
Green tea extract was provided by Guilan’s green tea leaves (250 g, Guilan Province, Islamic of Republic of Iran). In brief, after green tea powdered with an electric mill (JL-280, Zhengzhou Yize, China), 250 mL of 80% methanol (200 mL methanol + 50 mL of water) was added. Then, it was kept at the laboratory temperature (25°C - 28°C) for 24 hours. Finally, the extract was filtered (Whatman filter No.1) and dried at a temperature of 28°C - 30°C. The treatment groups received 1.34 mL GTE solution (10 mg GTE dissolved in 100 mL water) for each rat, three times per week for eight weeks by gavage (18, 20).
3.5. Tissue Preparation and Determination of VEGF, MMP-2/MMP-9 Proteins Levels
Rats were euthanized with ketamine (50 mg/kg) and xylazine (4 mg/kg) 48 hours after the last intervention session and subsequently, samples from prostate tissue were quickly removed and frozen in liquid nitrogen and stored at -80°C for further analyses. To extract total protein, 100 mg of prostate tissue was homogenized in microtubes containing 0.5 mM of solution containing phosphate-buffered saline (PBS) (pH = 7.4), anti-proteases PI cocktail (protease inhibitor) (Cas number 535140, Calbiochem), and PMSF (phenyl-methyl-sulfonyl fluoride) (CAS number 329-98-6; Merk, Germany). The tissue debris was removed by centrifugation at 1000 g for 12 min. Then the supernatant was used for evaluation of MMP-2, MMP-9, and VEGF by ELISA method according to the manual of the appropriate kits (ZellBio CmbH, Ulm, Germany). The rat specific kits, with the following specifications were used for this study: MMP-2 (Cat number: ZB-10315-R9648; sensitivity: 0.08 ng/mL; inter-assay precision: Cv% < 8%), MMP-9 (Cat number: ZB-10321-R9648; sensitivity: 0.01 ng/mL; inter-assay precision: Cv% < 8%) and VEGF (Cat number: ZB-10659-R9648; sensitivity: 0.5 pg/mL; inter-assay precision. Cv% < 8%).
3.6. Statistical Analysis
As the data had a normal distribution (Shapiro-Wilk and Levene’s tests), one-way ANOVA and Tukey post hoc tests were used to determine the significance of differences between variables. All statistical analysis was performed using SPSS version 20.0 software, and statistical significance was set at P value < 0.05. Data are expressed as mean ± standard deviation (SD).
4. Results
Changes in rats’ body weight during our study are presented in Table 1.
| Time | Body Weight, g | |||||
|---|---|---|---|---|---|---|
| Ctr | AT | GTE | AT + GTE | Sham | P Value | |
| Pre-test | P = 0.01b | |||||
| Healthy | 331.71 ± 16.50 | 326.55 ± 4.52 | 350.05 ± 7.31 | 325.55 ± 4.91 | 325.59 ± 5.17 | |
| Cancer | 306.28 ± 9.41 | 297.14 ± 14.00 | 287.85 ± 15.38 | 304.33 ± 17.33 | ||
| Post-test | P = 0.0001b | |||||
| Healthy | 410.25 ± 6.50 | 328.75 ± 2.18 | 380.35 ± 9.42 | 327.88 ± 6.14 | 405.20 ± 8.25 | |
| Cancer | 346.07 ± 12.84 | 366.57 ± 28.80 | 376.57 ± 24.71 | 333.33 ± 20.65 | ||
Abbreviations: AT, aerobic training; AT + GTE, aerobic training + green tea extract; Ctr, control; GTE, green tea extract.
aValues are expressed as mean ± SD.
bsignificant difference between groups.
The results revealed that body weight in the CaCtr, CaAT, CaGTE, and CaAT + GTE groups was lower than the healthy Ctr group (P = 0.0001, P = 0.001, P = 0.02, and P = 0.0001; respectively) at the beginning of the intervention. Also, body weight in the CaCtr, CaAT, CaAT + GTE groups and healthy AT, and AT + GTE groups was lower than the healthy Ctr group (P = 0.001, P = 0.01, P = 0.001, P = 0.001, and P = 0.006, respectively) after the end of the experimental period. In addition, body weight in CaAT + GTE group was lower than the CaGTE and CaAT groups after the intervention period (P = 0.001, and P = 0.004, respectively) (Table 1).
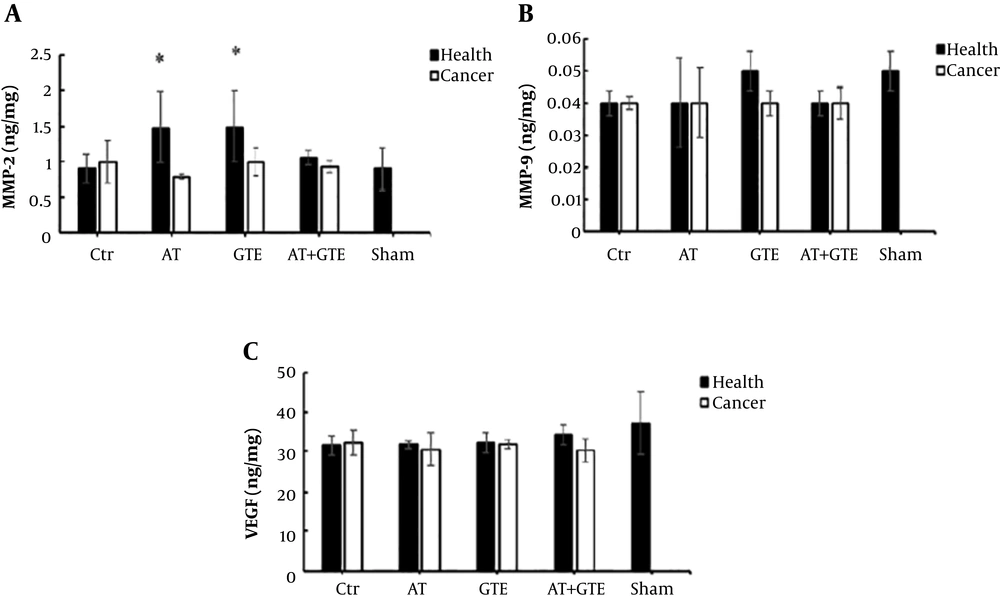
Based on the results, there was no significant difference between the healthy and CaCtr groups in the MMP-2 levels (P = 0.07), but MMP-2 level in the healthy AT and GTE groups was a tendency to increase compared to the healthy Ctr group (P = 0.11). However, the MMP-2 level in the CaAT group was lower than the healthy AT group (P = 0.01) (Figure 3A).
Effect of eight weeks aerobic training (AT) and green tea extract (GTE) on A: MMP-2 (matrix metalloproteinases-2); B: MMP-9 (matrix metalloproteinases-9); and C: VEGF (vascular endothelial growth factor) in prostate tissue. *, Significant differences compared with the CaAT group (P < 0.05). Ctr, control; AT, aerobic training; GTE, green tea extract; AT + GTE, aerobic training + green tea extract.
In addition, there was no significant difference in levels of MMP-9 (Figure 3B) and VEGF (Figure 3C) between the healthy and cancer groups after eight weeks of aerobic training and green tea extract consumption (P = 0.23 and P = 0.08, respectively).
5. Discussion
The findings of this investigation showed that MMP-2, MMP-9, and VEGF levels did not change after aerobic training and green tea extract consumption in the rats with prostate cancer and healthy rats. Also, the MMP-2 level had a trend to increase in the healthy aerobic training group compared to the healthy control group. In addition, the level of MMP-2 in the healthy aerobic training group was significantly greater than the cancer aerobic training group.
Exercise training through MMPs modulates the activation of cytokines, angiogenesis, and growth factors thus facilitate physiological adaptions (21). A previous study demonstrated that high-intensity interval exercise (HIIE) significantly decreases the MMP-2 level in persons with multiple sclerosis (22) and 12-weeks endurance training decreases MMP-9 concentration in persons at risk of coronary events (23). Morgia et al. (24) showed no significant difference in circulating MMP-2/-9 between sedentary healthy and participating in recreational physical activity subjects, while the result showed a reduction of MMP-9 level in the exercise breast cancer (BC) survivor group compared to sedentary BC survivors and elevate in MMP-2 circulating level in BC survivors. Giganti et al. (21) stated that the level of MMP-2/-9 can be modulated by various physical activities, and an exercise program may induce a different effect on the healthy or cancer groups. Jones et al. (25) investigated the effects of voluntary wheel exercise in mice with murine PrCa cells. Results showed that the expression of pro-metastatic genes in the exercise group was significantly reduced, which indicates a decline in metastasis and primary tumor growth rate. In pre-clinical models, PrCa proliferation was inhibited after four weeks of exercise training on a treadmill (26). Some epidemiological data, including in vitro and animal studies indicate that exercise training inhibits metastasis, angiogenesis, and prostate tumorigenesis in a dose-dependent manner (27). It has been suggested that VEGF and certain MMPs can be regulated by a mutually coordinated manner at a transcriptional level (9). Thus, a hypoxia-inducible factor (HIF-1) as a common master regulator causes simultaneously an expression of both VEGF and MMP-9 (9). Isanejad et al. (28) demonstrated that interval exercise training could down-regulate the expression of VEGF and HIF-1α that led to reducing angiogenesis. According to these results, exercise training may reduce tumor angiogenesis by affecting HIF-1α. On the other hand, Faustino-Rocha et al. (29) reported higher levels of VEGF expression after long-term exercise training in female rats with breast cancer. Based on previous studies, exercise training, especially endurance training has a dual effect on metastasis in both healthy and cancer patients. For instance, exercise training, especially endurance training increase angiogenesis factors in healthy subjects (4), but angiogenesis was decreased in tumor tissue in patients with cancer (30). Difference effects of exercise training on the process of angiogenesis indicate the effectiveness of training by activating or inhabiting mechanisms that influence the angiogenesis process in diseases.
In the present study, the MMP-2/-9 and VEGF levels did not change after taking the green tea extract in the healthy and cancer rats. It has been demonstrated an inhibitory effect of green tea on many types of cancer cells (31). EGCG has potent antioxidant properties that the maximum chemopreventive property of green tea has mainly been related to it and is the major component of green tea polyphenols (15). Previous studies demonstrated EGCG possible cancer-preventive activity and inhibition of multiple signaling pathways involved in metastasis (32). It has been shown that the green tea component, epigallocatechin-3-gallate, through inhibiting angiogenesis also exerts its antitumor activity (15, 33). Tumorigenesis for nourishing growing tumors and metastasis requires the development of new blood vessels (15). It has been reported that EGCG treated cells had decreased migration and invasive in mice with carcinoma cells, also reduced tumor sizes and inhibited angiogenesis (15). Demeule et al. (31) demonstrated that green tea was the most potent inhibitor of MMP-2 and MMP-9 that these results are not consistent with our findings. Another study also showed that a significant reduction in MMP-9 and VEGF levels in immune-impaired (athymic) male nude mice by supplementation with 0.5% of the green tea (34). This inhibition may partially be associated with the anti-angiogenic effect of EGCG. It has been demonstrated a down-regulate of the expression of VEGF that is a potent angiogenic protein and has chemotactic effects on vascular endothelial cells by EGCG (15). These differences probably arise from the amount of green tea consumption in the examined groups.
Alterations in MMP-2/-9 and VEGF concentrations may be valuable biomarkers to reflect the influence of exercise and anti-oxidant supplementation on metastasis and angiogenesis in prostate cancer. Nevertheless, the limited evidence available regarding the effects of aerobic training and green tea on the MMP-2/-9 and VEGF is caused that analysis of the current study’s findings is difficult. In contrast, Gueritat et al. (26) demonstrated that combination antioxidant supplementation (pomegranate juice) with exercise training prevented the antiproliferative activity, whereas pomegranate juice or exercise training decreased prostate tumor proliferation through the modulation of extracellular-signal-regulated kinase phosphorylation. In this context, the association of two preventive strategies may blunt the positive effects of single treatment and interferes with important reactive oxygen species (ROS)-mediated physiological processes such as anti-oxidant adaptations. Further studies must explore the effect of exercise training and antioxidant supplementation on tumor metastasis and angiogenesis in prostate cancer.
5.1. Conclusions
The present data demonstrated that low-to moderate-intensity aerobic training and treatment with a low dose of green tea extract did not change in angiogenesis and metastasis markers. The results of the present study pave the way for further research on modulation approaches for tumor metastasis and vascularization in responses to exercise training and antioxidant supplementation. However, the results obtained from our in vivo study may not be generalized to other solid or nonsolid tumors, which need further investigations.