1. Background
Urinary lithiasis is a disease known since ancient times, with a high prevalence in the general population, which has experienced an increase in recent decades (1). There has also been an increase in medical activities aimed at its treatment (2) and, consequently, in the costs associated with both the treatment itself and the sick leave secondary to the disease (3).
However, the medical treatment of urinary lithiasis has shown little evolution in recent decades. The significant improvements that have occurred in surgical treatment and extracorporeal lithotripsy have relegated it to the background (4).
The knowledge of epidemiological factors and biochemical alterations associated with urolithiasis could serve as a target for future interventions (5). In this way, we can prevent the formation of the lithiasis before it appears, instead of treating it once established.
On the other hand, there is a lack of homogeneity in the alterations reported by the literature, with a wide variation in the biochemical alterations found (6-11).
2. Objectives
The aim of the study was, therefore, to identify the biochemical alterations in patients with calcium oxalate lithiasis.
3. Methods
A cross-sectional study was carried out, consisting of the assessment of biochemical alterations that lithiasic patients presented. The sample selection was made consecutively, and the analysis was performed on the patients who attended the urolithiasis consultation (adult population) and who were diagnosed with oxalocalcic stones. All patients underwent a complete clinical evaluation.
The patients underwent a basal metabolic study, including analysis of urinary biochemical parameters, pH and urinary volume, as well as serum analysis of various parameters. A urine culture was performed to rule out concomitant urinary tract infection. All measurements were performed according to the standards of the hospital laboratory. Biochemical alteration was defined as a value outside the reference interval (95% of the population).
Patients under 18 years of age were excluded. Also, the appearance of nephritic colic can produce an alteration in the urinary and serum measurements due to the treatment (forced hydration, analgesics, etc.) Therefore, patients who had suffered from colic in the last month before the basal metabolic study were excluded from the study.
Regarding statistical analysis, categorical variables are expressed in absolute frequency and percentage, and quantitative variables as mean ± SD (standard deviation) or median (IQR, interquartile range). The Kolmogorov-Smirnov and Levene tests were used to verify the normality of the variable and the homogeneity of variances, respectively.
An analysis of the prevalence of each biochemical alteration in the sample of patients was performed. The comparison with the reference population was made by contrasting the proportion of patients who were above or below the reference interval with respect to the theoretical 0.025 using the binomial law. It was considered clinically relevant if the proportion of patients who were above or below the limits exceeded 0.10.
Body mass index (BMI) was categorized in patients not overweight (BMI < 25) and overweight (BMI > 25). Age was categorized as “young adult” (< 40 years old), “middle adult” (40 - 60 years old), and “elderly adult” (> 60 years old). The analysis of the different age categories in association with the presence of biochemical alterations was made by logistic regression (significance obtained with the likelihood ratio test). The comparative analysis of the parameters according to sex and BMI was performed using the Chi-square test (or Fisher’s exact test when appropriate).
The difference between men and women in terms of urine density, volume, and pH was calculated by student’s t-test (or Mann-Whitney U). These parameters were also studied according to BMI. The ANOVA test was used to assess urine density, pH, and volume in the different age groups. An analysis of vitamin D3 values was carried out to compare patients with PTH elevation with those who had normal levels using the student’s t-test.
All analyses were carried out assuming a level of significance of 5% (Bonferroni correction); the contrasts are bilateral. The analyses were performed with the statistical package SPSS (version 20.0, SPSS Inc., Chicago, IL).
The study was carried out in accordance with the code of ethics included in the Declaration of Helsinki, and with the current Spanish legislation. All patients were informed and signed the written informed consent. The study was approved by the Ethics Committee of Ramon y Cajal University Hospital, code 65/10.
4. Results
A total of 151 metabolic studies were performed. The median age of the patients was 51 years old (18.6 - 84.8). 32 (21.2%) were younger than 40 years old, 86 (57%) between 40 and 60, and 33 (21.9%) older than 60. 64.9% of patients were male. The mean age of women was 48.3 years-old (SD 14.9), and mean age of men 51.5 (SD 11.8). The mean BMI was 25.9 (SD 3.7) kg/m2. 81 patients (53.6%) were overweight.
The mean urinary volume was 1944.9 (SD 764.4) mL/24 h, and the mean urinary density was 1012, 4 (SD 5.7) mg/mL. The mean urinary pH was 5.74 (SD 0.58); 44.4% of the patients had a diminished pH and 6% had an elevated pH. The mean creatinine clearance was 91.4 (SD 20.0) mL/min.
Table 1 shows the biochemical alterations and their statistical significance according to binomial law. The percentage of patients with biochemical alterations in the study, including serum or urinary ions and PTH, was 100%. The measurement error of the 151 patients in the sample population is 0.5% (95% confidence interval).
| Percentage of Patients, % | P | |
|---|---|---|
| Urine parameters | ||
| Hypocitraturia | 84.7 | 0.000 |
| Hyperoxaluria | 11.8 | 0.000 |
| Elevated urinary urea | 18.7 | 0.000 |
| Decreased urinary urea | 5.3 | 0.036 |
| Hypercreatinuria | 6 | 0.014 |
| Hypocreatinuria | 0.7 | 0.107 |
| Elevated pH | 6 | 0.014 |
| Decreased pH | 44.4 | 0.000 |
| Hyperuricosuria | 2.6 | 0.523 |
| Hypouricosuria | 31.8 | 0.000 |
| Hyperphosphaturia | 11.9 | 0.000 |
| Hypophosphaturia | 21.2 | 0.000 |
| Hypercalciuria | 51.7 | 0.000 |
| Hypermagnesiuria | 3.3 | 0.327 |
| Hypomagnesiuria | 39.7 | 0.000 |
| Hypernatriuria | 10.7 | 0.000 |
| Hyponatriuria | 2.7 | 0.518 |
| Hyperkaliuria | 0.7 | 0.109 |
| Hypokaliuria | 4.7 | 0.084 |
| Serum parameters | ||
| Hypercreatininemia | 6 | 0.014 |
| Hypocreatininemia | 15.2 | 0.000 |
| Hyperuricemia | 4.6 | 0.086 |
| Hypouricemia | 13.9 | 0.000 |
| Hyperphosphatemia | 4 | 0.178 |
| Hypophosphatemia | 9.9 | 0.000 |
| Hypercalcemia | 9.3 | 0.000 |
| Hypocalcemia | 8.6 | 0.000 |
| Hypermagnesemia | 1.3 | 0.269 |
| Hypomagnesemia | 23.8 | 0.000 |
| Hypernatremia | 2 | 0.477 |
| Hyponatremia | 4.6 | 0.086 |
| Hyperkalemia | 1.3 | 0.269 |
| Hypokalemia | 0.7 | 0.107 |
| Hyperzinquemia | 44.2 | 0.000 |
| Hypozinquemia | 2.7 | 0.502 |
| Elevated PTH | 29.1 | 0.000 |
| Decreased PTH | 7 | 0.107 |
The percentage of patients who had only one biochemical alteration was 0.7%, two or three alterations 8.6%, four 20.5%, five 19.2%, and six or more alterations 42.5%.
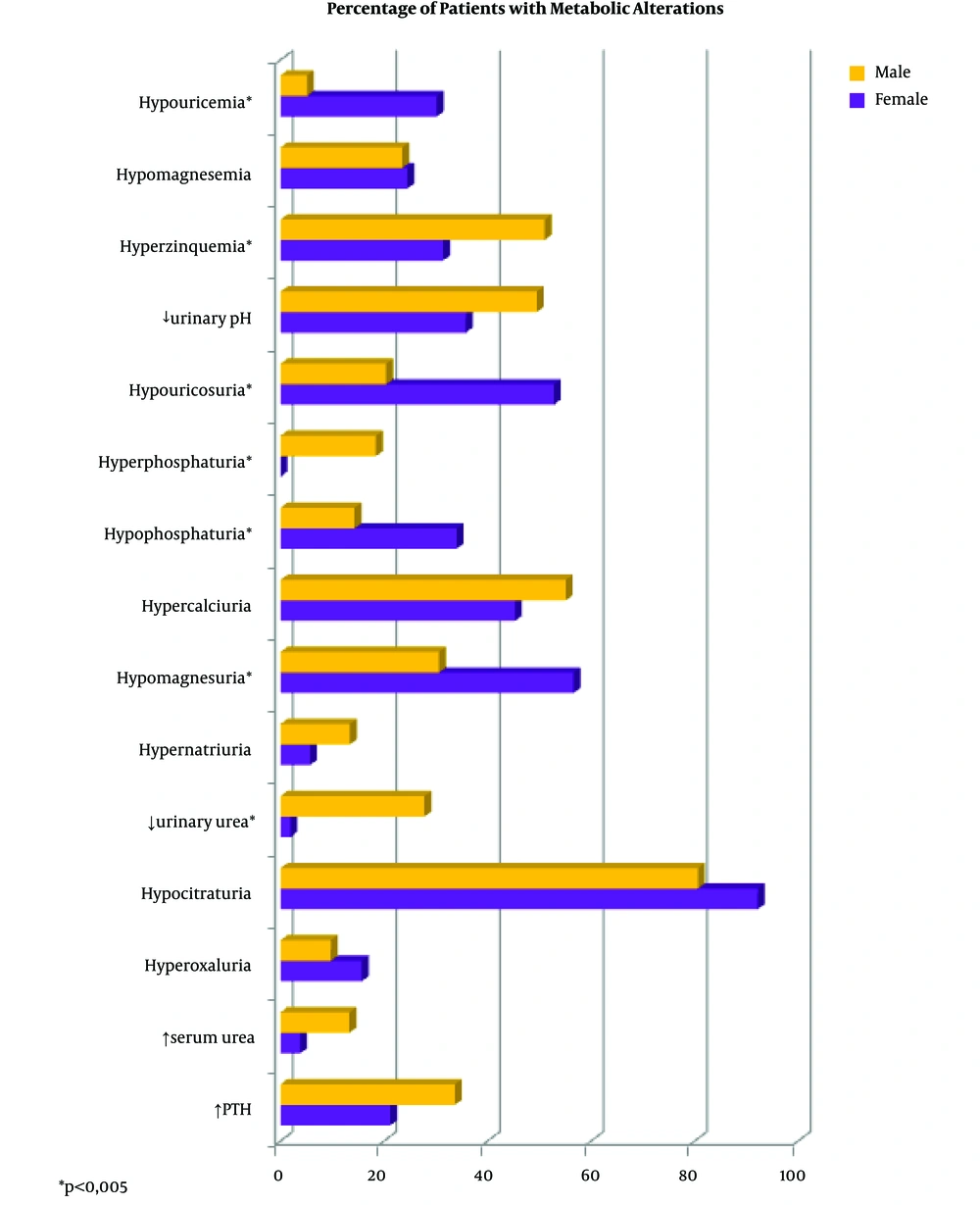
Figure 1 shows the percentage of patients who had biochemical alterations according to sex. Those with a statistically significant difference appear highlighted. A higher percentage of hypouricemia, hypouricosuria, hypophosphaturia, and hypomagnesuria were observed in women. Men had a higher percentage of hyperzinquemia, hyperphosphaturia, and excess of urinary urea.
The urine density in men was slightly higher (1013.1 mg/mL), than in women (1011.2 mg/mL, P = 0.008). Urinary volume was not statistically different between men and women (P = 0.842). The average pH in men was 5.69, and in women, it was 5.83, but this difference was not statistically significant.
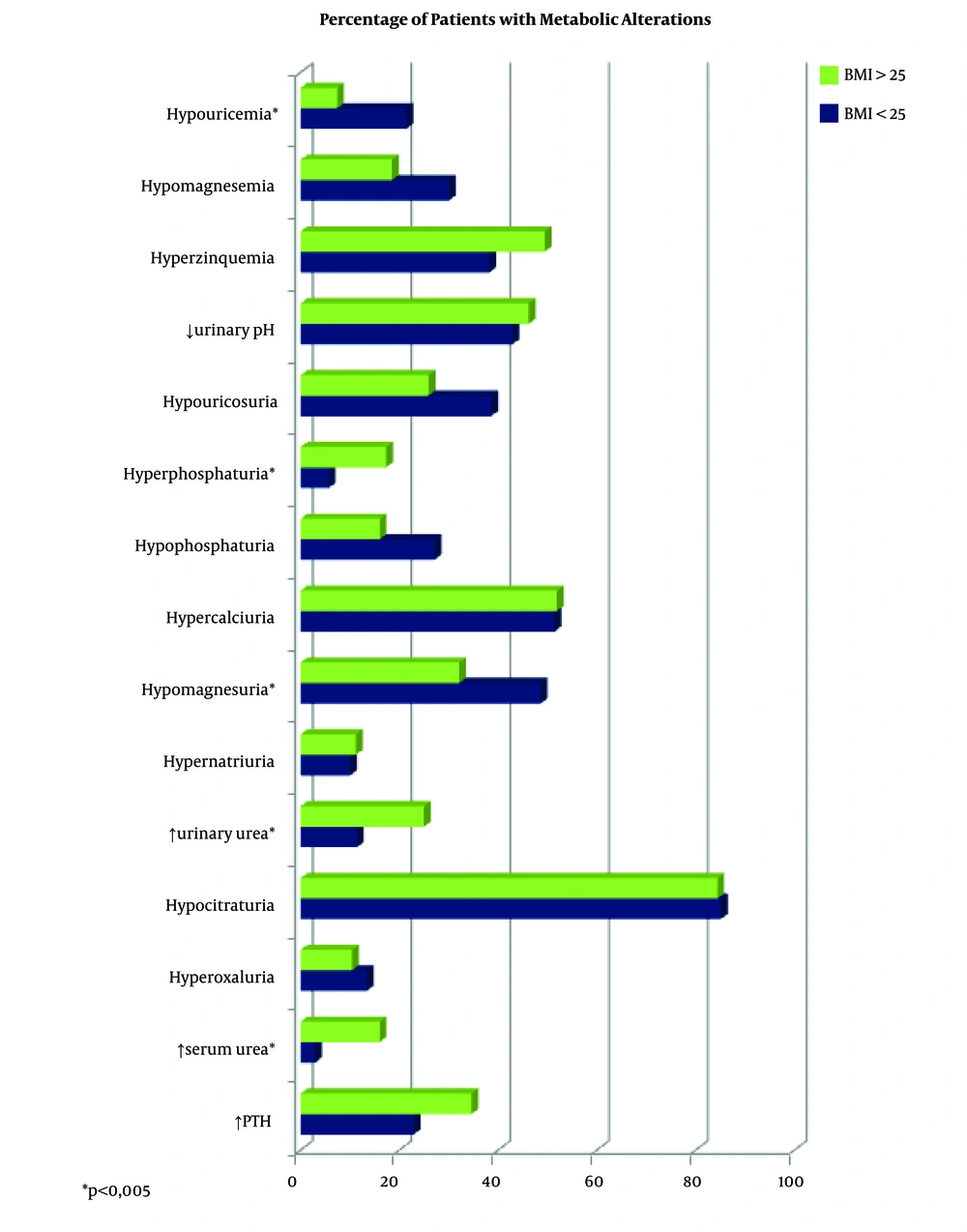
Biochemical alterations regarding BMI are shown in Figure 2. A higher percentage of hyperphosphaturia, excess of urinary urea, and excess of serum urea were observed in overweight patients. On the other hand, patients not overweight showed a higher percentage of hypouricemia and hypomagnesuria.
Overweight patients had a slightly higher urinary density than those not overweight (1,013.4 vs. 1011.3 mg/mL, P = 0.002). The volume was rather lower in overweight patients (1,845 vs. 2,060 mL/24 h), but this difference was not significant (P = 0.086). Patients not overweight had a slightly higher mean pH than those overweight (5.8 vs. 5.7), but it was not statistically significant.
Table 2 indicates the percentage of patients who presented biochemical alterations according to the age groups. No statistically significant differences were found in the biochemical alterations, according to age groups.
| Biochemical Alterations | Percentage of Patients with Biochemical Alterations | P | ||
|---|---|---|---|---|
| Young Adult (< 40 Years) | Middle Adult (40 - 60 Years) | Elderly Adult (> 60 Years) | ||
| Hypouricemia | 12.5 | 17.4 | 6.1 | 0.225 |
| Hypomagnesemia | 25 | 23.3 | 24.2 | 0.979 |
| Hyperzinquemia | 25.8 | 48.8 | 50 | 0.059 |
| ↓ Urinary pH | 46.9 | 45.3 | 40.6 | 0.864 |
| Hypouricosuria | 40.6 | 30.2 | 27.3 | 0.467 |
| Hyperphosphaturia | 12.5 | 10.5 | 15.2 | 0.780 |
| Hypophosphaturia | 25 | 18.6 | 24.2 | 0.670 |
| Hypercalciuria | 56.2 | 54.7 | 39.4 | 0.276 |
| Hypomagnesuria | 43.8 | 34.9 | 48.5 | 0.349 |
| Hypernatriuria | 9.4 | 12.8 | 6.2 | 0.549 |
| ↑ Urinary urea | 15.6 | 19.8 | 18.8 | 0.873 |
| Hypocitraturia | 87.1 | 86.9 | 75.9 | 0.367 |
| Hyperoxaluria | 16.1 | 9.5 | 13.8 | 0.589 |
| ↑ Serum urea | 9.4 | 8.2 | 15.2 | 0.554 |
| ↑ PTH | 18.8 | 29.1 | 39.4 | 0.182 |
Urinary volume was higher in the middle-aged patients (2085.4 mL/24 h) than in young patients or those over 60 years (1699.1 and 1817.3 mL/24 h. respectively). There were no statistically significant differences in terms of urinary pH and density by age groups.
Patients with high PTH showed values up to a maximum of 100 pg/mL. except from 3. The highest of the latter reached a value of 122.6 pg/mL. In patients who had an increase in PTH, the average vitamin D3 was 17.74 ng/mL (below the lower limit for vitamin D3). In those without PTH increase, the mean value of vitamin D3 was 23.08 (P = 0.136) ng/mL. Although the mean levels of vitamin D3 were lower in those patients who had elevated PTH, differences were not statistically significant.
5. Discussion
In the presented study, sex distribution was similar to what is published in the literature for lithiasic patients in most studies, where the prevalence in men is usually reported two times higher than in women (6). The ages of the patients in this study were distributed between 18 and 84 years, but most were within the intermediate ages of life, forming a normal distribution. Our data coincide with the distribution reported by the literature (6, 7).
Moreover, 53.6% of the patients presented overweight. The association between urolithiasis and obesity is well known (12-15), and is likely to be one of the risk factors for the increase in the incidence of lithiasis in developed countries.
The percentage of urolithiasic patients with biochemical alterations ranges between 90.5% and 96.8% in the literature (8-10, 16-18). In addition, usually, more than one biochemical alteration was found in each patient (8-10, 16-18). In our study, 100% of the patients presented biochemical alterations. The most frequent one was hypocitraturia (84.7%), followed by hypercalciuria (51.7%), and a decreased urinary pH (44.7%).
The role of citrate is fundamental in the genesis of urolithiasis, since it has been observed that crystallization decreases with citrate, so it potentially has a protective role for all types of lithiasis. The majority of the studies coincide in a decrease of urinary citrate in patients with urolithiasis, ranging from 10.55% to 57.2% (8, 9, 16, 17, 19, 20). This circumstance has been used to treat patients with substances rich in citrate, and this has become one of the most used medical treatments.
Hypercalciuria was the second metabolic disorder in frequency, reaching 51.7%. This data is in the range of hypercalciuria that is described in the literature, between 14.5% and 74%. Hypercalciuria has been associated with an increase in the formation of urinary stones, both calcium oxalate and calcium phosphate, as well as mixed (21-24).
The decrease in pH was the third most frequent biochemical alteration in the patients. Although the presence of alterations in pH in lithiasic patients has previously been described, the proportion is usually lower than in the study (16).
Hypomagnesuria, with 39.7% of subjects, was the fourth metabolic disorder in frequency. The decrease in urinary magnesium is also a frequent finding in metabolic studies performed in patients with urinary lithiasis, ranging from 12.9% to 30.7%. This may be due to the fact that magnesium forms complexes with oxalate, thereby reducing the supersaturation of calcium oxalate. In addition, magnesium oxalate complexes reduce the intestinal absorption of oxalate. At physiological concentrations of oxalate, magnesium reduces both nucleation rates and the growth of calculus (8, 9, 16-18).
Hypouricosuria was the fifth metabolic disorder in the frequency of this study, being present in 31.8% of patients. This data is striking since hyperuricosuria is a risk factor classically associated with the formation of urinary stones, not only uric lithiasis, but also oxalocalcic lithiasis. However, in this study, hyperuricosuria was present in only 2.6% of patients. In the literature, the percentages of hyperuricosuria range between 12.9% and 30.7% (8, 9, 16-20). This higher percentage may be explained because the referred studies comprise all types of stones, including uric acid stones, which are more frequently associated with hyperuricosuria, unlike the present study, which is limited to calcium oxalate lithiasis.
Hyperoxaluria was present in 11.8% of our patients. The presence of hyperoxaluria reported in the literature ranges between 2.6% and 64.1% (8, 10, 16-20, 25), being the most frequent biochemical alteration found in some cases (10).
Also, 11.9% of the patients had hyperphosphaturia. This finding correlates with what has been previously published. The reduction of renal reabsorption of phosphates takes place basically at the level of the proximal tubule and is closely related to the reabsorption of sodium through the sodium-phosphate cotransporter so that phosphaturia is favored with the ingestion of diets rich in sodium (17). In this study, 10.7% of the patients presented an elevation of urinary sodium. The association of urinary sodium with urinary volume, pH, Oxalate, and citrate has also been described in the literature (9, 26, 27).
With regard to urinary volume, the observed median of 1.860 mL/24 h can be considered high. These findings can be explained because of the popular knowledge in lithiasic patients of an abundant intake of fluids. Urinary volume was not statistically different between men and women (P = 0.842), which contrasts with what is published by other authors (25), who observed a lower diuresis in women. Nevertheless, the decrease in diuresis has been reflected in the literature as a clear lithogenic risk factor by increasing the relative concentration of solutes in the urine (28).
Urinary density and osmolarity vary in a parallel manner, while there is no high molecular weight substance in the urine. In this study, urine density was evaluated. It was observed that urine density in men was slightly higher (1013.1 mg/mL), than in women (1011.2 mg/mL), this difference being statistically significant. This is consistent with that published by Perucca et al. (29), who describes an increase in men’s urine osmolality with respect to women.
In this study, a significant percentage (29.1%) of patients with parathormone levels above the upper limit of the reference interval was found. In these patients, the concentration of vitamin D3 was analyzed secondarily. It was observed that they had, on average, lower levels of vitamin D3 than patients without PTH elevation; however, the difference was not statistically significant. This deficit of vitamin D3 could lead to a decrease in serum calcium, which reactivates serum levels of PTH as a compensatory way to raise serum calcium (30, 31).
Different prevalence of lithiasis has been described by both sex and age (32-34). In order to explain this circumstance, the metabolic differences existing in the different populations have been studied. Lancina Martin et al. (35) described a greater presence of hyperoxaluria, hyperuricosuria and hypocitraturia in men and a higher presence of hypercalciuria and decreased urinary volume in women. This lower presence of hyperoxaluria in women may be due to the effect of oestrogen. Ferraro et al. (20), in contrast, found a lower urinary calcium excretion in women, but also a reduction in the excretion of uric acid (20, 35). Another study describes the changes that have occurred in women in recent times, confirming an increase in the excretion of oxalate and urinary calcium, and a decrease in urinary magnesium. However, an increase in the urinary excretion of citrate was also found. The excretion of phosphorus and uric acid decreased. Calcemia decreased, whereas serum phosphorus and magnesium increased, and serum uric acid did not show any variation. The authors of the study relate these changes in the parameters with the modifications in the lifestyle of recent years (36). Sanchez-Martin et al. (25) in 2017, published a review on differences in metabolic parameters by sex. A greater excretion of calcium, phosphorus, magnesium, oxalate and uric acid was observed in men and a greater excretion of citrate in women. On the other hand, it should be noted that there are papers that describe the absence of metabolic differences between sexes (19).
In the present study, it was observed that women had a higher percentage of hypouricemia, Hypouricosuria, hypophosphaturia and hypomagnesuria. Higher percentages of hyperzinquemia, Hyperphosphaturia, and excess urinary urea were found in men. Besides, urine density in men was slightly higher (1013.1 mg/mL), than in women (1011.2 mg/mL, P = 0.008). The findings in this regard are very heterogeneous, and no conclusions can be drawn regarding the relationship of sex with biochemical alterations. Therefore, the relevance of some of these values in the development of urolithiasis remains to be determined. No specific analysis was performed on menopausal women as the sample was small (9 older than 60, 15 between 50 and 60 years old). Some authors have found different patterns in this group of patients (37).
Regarding age, a greater presence of hyperoxaluria, hyperuricosuria, and decreased urinary volume in patients older than 60 years are described in the literature, but with a higher prevalence of biochemical alterations in those under 60 years of age. Besides, a lower hypocitraturia in older women is reported. Other authors found that increases in age lead to an increased BMI and also decreased urinary pH, calcium, uric acid, ammonium, and creatinine, as well as a supersaturation of calcium oxalate and calcium phosphate. In a study with young patients, a higher prevalence of hypercalciuria and hypocitraturia was observed (38-40). In contrast, an increase in hypercalciuria, hyperuricosuria and increased calcium oxalate saturation in patients under 60 years old has also been described. Also, a decrease in urine volume in patients under 40 years is reported (35). In this study, the only difference found was a higher urinary volume in middle-aged patients. Therefore, also, in this case, the findings are disparate and do not allow us to draw clear conclusions regarding the biochemical alterations between different ages and their influence on lithogenesis.
With regard to BMI, overweight patients had a higher percentage of hyperphosphaturia, excess urinary urea, hyperglycemia and excess of serum urea. On the other hand, patients not overweight showed a higher percentage of hypouricemia and hypomagnesuria. In the published literature, a higher prevalence of biochemical alterations has been found in obese patients, with an increase in the excretion of lithiasis promoters (oxalate, calcium, uric acid), and a decrease in pH. With respect to the excretion of lithogenesis inhibitors (citrate), in some papers, an increase of excretion is described in overweight patients, while a decrease of citrate is shown in others (15, 41-43).
Regarding the limitations of the study, a single determination was made in the 24-hour urine. However, there are groups that recommend performing two separate determinations in time (8, 44). Parks et al. (45) observed that the metabolic characteristics of the patients varied according to the season of the year in which the metabolic study was carried out. In the present study, the season of the year in the assessment of the metabolic study has not been taken into consideration. Neither has been the influence of diet.
On the other hand, one study performed a urinalysis to patients with oxalocalcic lithiasis at various times throughout the day, observing that the pH oscillated, such that it decreased in the morning, increased at midday and returned to decrease at night (46). In this study, the possible variations of the pH throughout the day have not been taken into account since the pH analysis was carried out in fresh urine, always collected in the morning between 8.30 and 10 h.
There are research groups that, in the basal metabolic study of patients with urolithiasis, include a urine acidification test in order to identify those with renal tubular acidosis (8). In the current study, a urinary acidification test was not performed. However, this disorder is frequently associated with calcium phosphate stones, which are not the ones studied in this case. Other parameters analyzed occasionally in metabolic studies are urinary saturation indexes, both calcium oxalate, and calcium phosphate or other substances (47). In this study, the saturation indices have not been analyzed.
Also, despite some groups that have assessed metabolic differences between calcium oxalate monohydrate and dehydrate lithiasis (48, 49), in this study, they were considered altogether. Nevertheless, stones with other minor components that had different patterns in some studies (50) were excluded.
5.1. Conclusions
Patients with oxalocalcic lithiasis had biochemical alterations in 100% of the cases. The most frequent were hypocitraturia (84.7%), hypercalciuria (51.7%), and urinary pH decrease (44. 4%). These biochemical alterations varied according to sex, age, and BMI. Considering these data, it is advisable to perform a metabolic evaluation of patients with urolithiasis to carry out interventions to reduce recurrences.