1. Background
Chronic kidney disease (CKD) occurs in 10% to 16% of the global population (1, 2). The prevalence of chronic kidney disease is increasing in developed and developing countries (1). This increase is much more eminent in low- and middle-income countries, where the annual increase in mortality due to CKD is estimated to be more than 5% (3). The economic burden of CKD is hard to calculate as CKD is associated with a variety of indirect treatment costs and harms that may not be easily taken into the calculation (4). Renal replacement therapy is the only life-saving treatment for end-stage renal disease (5). Renal replacement therapy includes hemodialysis (HD), continuous ambulatory peritoneal dialysis (CAPD), and renal transplantation (5).
Increased pulmonary artery blood pressure is defined as pulmonary artery hypertension (PAH) (6). PAH is defined as pulmonary artery pressure (PAP) above 25 mmHg based on angiography and 35 mmHg based on echocardiography measurements (7). PAH is a chronic progressive disease that may result in right ventricular heart failure and sudden death (6, 8). The underlying causes of PAH include heart and lung conditions, thromboembolic diseases, and systemic diseases, including rheumatoid arthritis as well as conditions involving the liver (6). PAH is also frequent among patients with chronic kidney disease (9% - 39% in chronic kidney disease classes) and hemodialysis (18.8% - 68.8%), and CAPD (0% - 42%) (9-11).
Furthermore, PAH is associated with an increased risk for cardiovascular complications in HD patients (12). The risk factors for PAH in HD patients include systolic and diastolic dysfunction, age, smoking, urea reduction rate, and vitamin D receptor activator use (10, 13). It is also hypothesized that secondary hyperparathyroidism might result in PAH in HD patients due to vascular calcification, but the results of previous studies regarding the effect of parathyroid hormone (PTH) on PAH are controversial (13-16).
2. Objectives
This study was aimed to assess the association between PAH and PTH among patients undergoing regular HD.
3. Methods
This was a cross-sectional study conducted among HD patients who visited a tertiary hospital in Mashhad, Iran. This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (reg. no.: IR.MUMS.MEDICAL REC1397.156). All the patients signed a written informed consent before beginning the study.
The inclusion criteria were age above 18 years old, being on regular dialysis for at least 6 months, and willingness to participate in the study. Patients with other diseases or conditions that affected PAP were excluded from the study.
All the patients underwent echocardiography by a trained echocardiography subspecialist. All the echocardiographic assessments were performed by a single subspecialist. Patients were categorized into PAH and non-PAH based on the measured PAP and the previously defined cut off for PAH (PAP > 35 mmHg) (7). Ejection fraction (EF) was also measured and recorded for each patient.
Laboratory measurements were extracted from the latest laboratory assessments in patient documents and included serum calcium, phosphorus, PTH, vitamin D, albumin (Alb), alkaline phosphatase (Alp), and hemoglobin (Hb).
3.1. Statistical Analysis
SPSS version 21.0 (IBM Inc, Chicago, Il, USA) was used for data analysis. Normality distribution was assessed by performing the Shapiro-Wilk test. A comparison of variables between the groups was performed using independent t-test and Mann Whitney U-test for normally distributed and non-normally distributed variables, respectively. Categorical variables were described using the chi-squared test. The relationship between pulmonary hypertension and hyperparathyroidism was assessed using logistic regression analysis before and after adjustment for other study variables. Logistic regression results were presented using P value, odds ratio (OR), and 95% confidence interval (CI) for OR. The statistical significance level was considered as P < 0.05.
4. Results
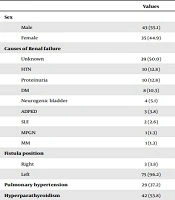
Seventy-eight patients (43 males and 35 females) were enrolled in this study. The mean age was 37.25 ± 11.98 years. The demographic characteristics of the study patients are presented in Table 1. PAH was present in 29 (37.2%) patients, while hyperparathyroidism was present in 42 (53.8%) patients. The most common causes of renal failure among the patients were unknown (50.0%), hypertension (12.8%), proteinuria (12.8%), and diabetes mellitus (10.3%).
| Values | |
|---|---|
| Sex | |
| Male | 43 (55.1) |
| Female | 35 (44.9) |
| Causes of Renal failure | |
| Unknown | 39 (50.0) |
| HTN | 10 (12.8) |
| Proteinuria | 10 (12.8) |
| DM | 8 (10.3) |
| Neurogenic bladder | 4 (5.1) |
| ADPKD | 3 (3.8) |
| SLE | 2 (2.6) |
| MPGN | 1 (1.3) |
| MM | 1 (1.3) |
| Fistula position | |
| Right | 3 (3.8) |
| Left | 75 (96.2) |
| Pulmonary hypertension | 29 (37.2) |
| Hyperparathyroidism | 42 (53.8) |
Abbreviations: ADPKD, adult polycystic kidney disease; DM, diabetes mellitus; HTN, hypertension; MPGN, membranoproliferative glomerulonephritis; MM, multiple myeloma; SLE, systemic lupus erythematosus.
aValues are expressed as No. (%).
The distribution pattern of PAH among the hyperparathyroidism group was compared using the Chi-squared test. There was a significant difference in the distribution pattern of PAH between hyperparathyroidism and normal PTH groups (P = 0.003).
No significant difference was observed in the study parameters between patients with PAH and patients with normal pulmonary artery pressure (P > 0.05; Table 2).
| PAH | Number | Values | P | |
|---|---|---|---|---|
| Age, y | No | 49 | 36.90 ± 11.48 | 0.743 |
| Yes | 29 | 37.83 ± 12.97 | ||
| Ca, mg/dL | No | 49 | 8.92 ± 0.89 | 0.505 |
| Yes | 29 | 8.78 ± 0.96 | ||
| P, mg/dL | No | 49 | 5.39 ± 1.59 | 0.768b |
| Yes | 29 | 5.49 ± 1.37 | ||
| Alb, g/dL | No | 49 | 4.17 ± 0.48 | 0.184 |
| Yes | 29 | 4.02 ± 0.46 | ||
| PTH, pg/mL | No | 49 | 348.45 ± 311.87 | 0.103b |
| Yes | 29 | 464.89 ± 331.61 | ||
| Vitamin D, ng/mL | No | 49 | 30.66 ± 7.85 | 0.660b |
| Yes | 29 | 30.38 ± 9.22 | ||
| Alp, IU/L | No | 49 | 329.96 ± 304.84 | 0. 079b |
| Yes | 29 | 388.86 ± 238.23 | ||
| Hb, g/dL | No | 49 | 11.70 ± 1.64 | 0.169 |
| Yes | 29 | 11.19 ± 1.43 | ||
| EF, % | No | 49 | 52.80 ± 4.12 | 0.427b |
| Yes | 29 | 52.07 ± 5.26 |
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; Ca, calcium; EF, ejection fraction; Hb, hemoglobin; P, phosphorus; PAH, pulmonary artery hypertension; PAP, pulmonary artery pressure; PTH, parathyroid hormone.
aValues are expressed as mean ± SD.
bThe Mann-Whitney test was used for the comparison due to non-normal distribution of the data.
There was a significant association between hyperparathyroidism and pulmonary hypertension based on the chi-squared test (Table 3). Logistic regression revealed that PAH was significantly related to hyperparathyroidism (P = 0.004, OR = 4.557, 95% CI for OR = 1.637, 12.684). There was a significant relationship between hyperparathyroidism and age (P = 0.033, OR = 0.944, 95% CI for OR = 0.895, 0.995) and serum calcium level (P = 0.005, OR = 0.336, 95% CI for OR = 0.156, 0.720) . After adjusting for other variables including calcium and age, the odds ratio for the relationship between pulmonary hypertension and hyperparathyroidism increased from 4.557 to 7.593, indicating that after adjusting for confounders, the odds of having hyperparathyroidism in the presence of pulmonary hypertension increases by 7.593 times compared to normal pulmonary artery pressure (Table 4).
| Variable | P | OR | 95% CI for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Pulmonary hypertensionb | ||||
| Unadjusted | 0.004c | 4.557 | 1.637 | 12.684 |
| Adjusted | 0.003c | 7.593 | 2.033 | 28.362 |
| Ca | 0.005c | 0.336 | 0.156 | 0.720 |
| P | 0.408 | 0.848 | 0.573 | 1.254 |
| Alb | 0.511 | 1.561 | 0.414 | 5.884 |
| Alp | 0.728 | 1.000 | 0.998 | 1.003 |
| Hb | 0.261 | 1.251 | 0.847 | 1.850 |
| EF | 0.463 | 0.949 | 0.827 | 1.091 |
| Vitamin D | 0.476 | 0.974 | 0.906 | 1.047 |
| Age | 0.033c | 0.944 | 0.895 | 0.995 |
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; Ca, calcium; CI, confidence interval; EF, ejection fraction; P, phosphorus; PTH, parathyroid hormone; PAP, pulmonary artery pressure; Hb, hemoglobin; OR, odds ratio.
bRelationship between pulmonary hypertension and hyperparathyroidism was assessed as univariable (unadjusted) and multivariable (adjusted for other variables) models.
cSignificant at α = 0.001.
5. Discussion
The findings of this study revealed that PAH was present in 37.2% of the HD patients. This finding was in line with the results of previous studies in different regions (12-16). One possible reason for the discrepancy in observations might be the difference in the cut off value for PAP to detect PAH. In a study in Iran among 62 HD patients, the prevalence of PAH was reported 62.3% based on a similar PAP cut off used in our study (17). However, the reported prevalence of PAH was higher than the observed prevalence of PAH in our study. One reason for this difference might be the difference in the age of the subjects as our patients were younger than the mentioned study.
This study also found that the most prevalent cause of CKD was unknown, followed by hypertension, proteinuria, and diabetes mellitus. This finding was in contrast to the findings of a previous study in the same province that reported hypertension and diabetes as the most common etiologies of CKD in 2404 HD patients (18). Similarly, in a study of 633 HD patients in Shiraz, hypertension, and diabetes were reported as the most common causes of CKD (19). The reason for this difference might be due to the larger sample size in the mentioned study. As our study was not designed to determine the prevalence of CKD etiologies, the shortcoming of our study in determining the prevalence of the etiology of CKD was not a limitation. On the other hand, this finding might affect the generalizability of the findings of this study to the whole HD population.
The findings of this study revealed that the prevalence of PAH was higher among hyperparathyroidism patients than those with normal parathyroid function. This study also showed a significant relationship between PAH and PTH levels. In a study of HD patients, PTH was significantly lower in patients with PAH and vascular calcification (16). Our study results were in contrast with the findings of recent studies (11, 15, 20). In a study among 77 HD patients in Turkey, no significant relationship was observed between serum PTH level and PAH (15). In another study of 119 HD patients in Brazil, no significant relationship was observed between PTH and PAH (20). In our previous study among 30 HD patients, no statistically significant relationship was observed between PTH and PAH (11). In another study of 69 HD patients, no significant relationship was found between PAH and PTH (17). Although these findings were in contrast to the present findings, in two studies that assessed arterial calcification, a significantly higher calcification was observed in patients with PAH regardless of the lack of a relationship between PAH and PTH levels (17, 21).
One of the limitations of this study was relying on serum markers for PAH. Future studies should focus on other possible indicators of PAH, including vascular calcification or stiffness. Studies with larger sample sizes and using a unified cut off for the detection of PAH are needed to better identify the relationship between PAH and PTH. Another limitation of this study was the inclusion of young HD patients in the study. As patient age might affect PAP and regarding the fact that chronicity of dialysis might also affect PTH, further studies with stratified sampling are required to assess the relationship between PAH and PTH in different age groups. The strength of this study was its sample size, which was larger than most published papers.