1. Background
Nephrotic syndrome is the most common glomerular disease affecting children worldwide (1). It is characterized by massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema, with normal renal function tests and no evidence of secondary causes of nephrotic syndrome (2, 3). Idiopathic, hereditary, and secondary forms are due to underlying increased protein leakiness across the glomerular capillary wall, as a result of immune and non-immune insults affecting the podocyte (3).
Prednisolone is the mainstay treatment, whose response is often presumed to determine the long-term risk of disease progression and is a better prognostic indicator (2, 4). Although patients with steroid-sensitive nephrotic syndrome generally have good outcomes, more than half will have a frequently relapsing course and steroid dependence. Up to 95% of patients with minimal change nephrotic syndrome (MCNS) attain complete remission after an 8-week course of high dose steroids (2).
The response to treatment with steroids has been shown to vary by ethnicity, likely due to environmental and genetic factors (5, 6). Studies in developed countries show good response to steroids in childhood nephrotic syndrome, with resistance seen more in Hispanics and Blacks (2, 4, 7). In Africa, nephrotic syndrome secondary to infections was previously considered predominant, with most reports favoring non-MCNS forms and lower steroid response rates (4, 8-10). However, studies in Southwest and Enugu state Nigeria reported a high steroid responsiveness of 82.5% and 85.7%, respectively (11, 12). In Tikur Anbessa Hospital, Ethiopia, 76.3% of all nephrotic syndrome patients had steroid-sensitive disease (13).
Idiopathic nephrotic syndrome occurs at a median age of 4 years, with older age of onset associated more commonly with secondary nephrotic syndrome and steroid resistance (14, 15). Nephrotic syndrome is more common in males, who also tend to achieve remission faster (4, 14). Females, on the other hand, respond late and have a higher risk of steroid resistance (16). Hypertension, macroscopic hematuria, and decreased GFR, which are atypical features of nephrotic syndrome, occur more in secondary forms and are predictors of steroid resistance (2, 9, 14, 16, 17). Currently, an increasing trend of focal segmental glomerulosclerosis with concomitant increase in steroid resistance has been shown by studies in Caucasians, Asians, and as well as Africans (8, 18-20). Although studies exist in various regions elucidating the pattern of response to prednisolone in nephrotic syndrome patients, there are no studies in the Tigray region, Northern-Ethiopia.
2. Objectives
To assess the pattern of response to prednisolone in pediatric patients treated for nephrotic syndrome at Ayder Comprehensive Specialized Hospital from 2014 to 2019.
3. Methods
3.1. Study Area, Period, and Design
A record-based retrospective study was carried out in Ayder Comprehensive Specialized Hospital, the second-largest referral hospital in Ethiopia, over 5 years (2014 to 2019).
3.2. Study Population
Eighty-one patients, aged 1 - 18 years, were diagnosed with nephrotic syndrome at Ayder Comprehensive Specialized Hospital within the study period. However, eighteen patients were excluded from the study. Diagnosis of nephrotic syndrome was made in the presence of edema, proteinuria of 3+ or 4+ on albustix testing, and hypoalbuminemia (serum albumin < 2.5 mg/dL).
3.3. Study Variables
Data was retrieved from both inpatient and outpatient charts via a predesigned form, which included age, gender, and laboratory investigations such as urinalysis, blood urea nitrogen, creatinine level, cholesterol, triglyceride levels, and serum albumin level. Other tests done were hepatitis B and C, human immunodeficiency virus, anti-nuclear antibody and/or anti-double-stranded DNA, and biopsy. Hypertension was defined based on the patient’s blood pressure measurements compared to percentiles for age, sex, and height based on the National High Blood Pressure Education program (NHBPEP) guidelines (21).
Patients with acute kidney injury were categorized into three stages according to the level of rise in creatinine above the baseline value for age (3). Response to prednisolone was classified according to kidney disease improving global outcomes; failure to achieve remission after eight weeks of steroids were categorized as steroid-resistant nephrotic syndrome (SRNS), while complete remission was considered steroid-sensitive nephrotic syndrome (SSNS). Patients were classified as frequently relapsing nephrotic syndrome (FRNS) if there were two or more relapses within six months of initial response or four or more relapses in any 12-month period. Patients with only one relapse in six months or less than 3 relapses in one year had infrequently relapsing nephrotic syndrome (SSNS). Steroid-dependent nephrotic syndrome comprised of two consecutive relapses during steroid taper, or within 14 days of terminating therapy (22).
3.4. Statistical Analysis
Socio-demographic, clinical, and biochemical characteristics were described by the number of patients and percentages. Binary logistic regression was used to test associations between patient characteristics and the response to prednisolone. Multivariate logistic regression was then used to identify independently associated variables with 95% confidence intervals. P value at < 0.05 was the cut off point for statistically significant association. Model fit was assessed by the Hosmer-Lemeshow test.
3.5. Ethical Clearance
Ethical clearance was obtained from the Ethical Review Board of Mekelle University, and an official letter of support was submitted to Ayder Comprehensive Specialized Hospital. Confidentiality was maintained throughout the study process.
4. Results
The patients’ age ranged from 1 - 17 years, with a median age of onset of three years. Age 7 - 12 years accounted for the most common age category of patients who were treated for nephrotic syndrome. The males predominated, constituting 81% of all patients, with a male to female ratio of 4.2:1. (Table 1).
| Socio-Demographic Characteristics | Values |
|---|---|
| Patients’ age | |
| 1 - 2 | 2 (3.2) |
| 2 - 6 | 18 (28.6) |
| 7 - 12 | 25 (39.7) |
| > 12 | 18 (28.6) |
| Total | 63 (100) |
| Patients’ sex | |
| Male | 51 (81) |
| Female | 12 (19) |
| Male:female ratio | 4.2:1 |
| Median age, years | 3 |
aValue are expressed as No. (%).
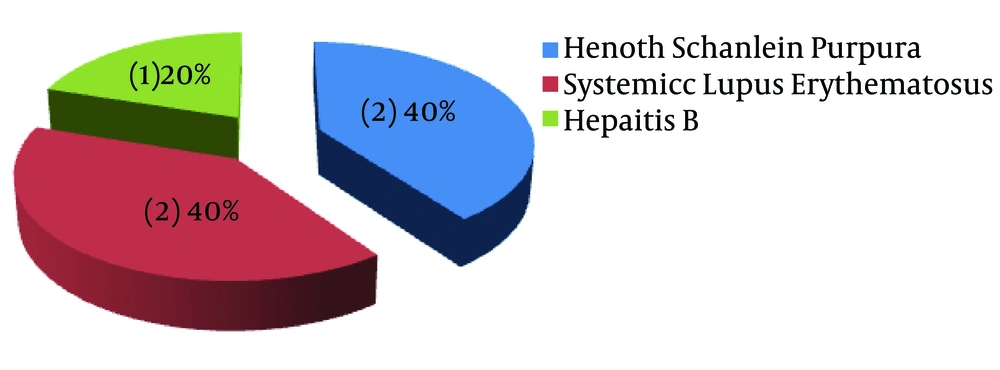
Hematuria was present in 42 patients (66.7%), among whom twenty-eight cases (66.7%) had gross hematuria. Hypertension was found in 31 patients (49.2%), whereas acute kidney injury was recorded in 11 patients (17.5%). One patient had a biopsy done due to failure to respond to steroid therapy, which revealed FSGS. Secondary causes of nephrotic syndrome were identified among five patients (Table 2). Secondary nephrotic syndrome was attributed to Henoch Schonlein Purpura, systemic lupus erythematosus, and hepatitis B virus (Figure 1).
| Patients’ Clinical Characteristics | Values |
|---|---|
| Hematuria | |
| Patients with hematuria | 42 (66.7) |
| Without hematuria | 21 (33.3) |
| Type of hematuria | |
| Gross hematuria | 28 (66.7) |
| Microscopic only | 14 (33.3) |
| Hypertension | |
| Hypertensive | 31 (49.2) |
| Non-hypertensive | 32 (50.8) |
| Stage of hypertension | |
| Stage 1 | 6 (18.2) |
| Stage 2 | 27 (81.8) |
| Acute kidney injury | |
| Patients with AKI | 11 (17.5) |
| Patients without AKI | 52 (82.5) |
| Stage of AKIa | |
| Stage 1 | 0 (0.0) |
| Stage 2 | 2 (20.0) |
| Stage 3 | 8 (80.0) |
| Biopsy | |
| FSGS | 1 (1.5) |
| No Biopsy | 62 (98.5) |
| Secondary causes of NS | |
| Secondary NS | 5 (8) |
| None | 58 (92) |
aValue are expressed as No. (%).
bAKI: stage 1, 1.5 - 1.9 times baseline creatinine; stage 2, 2.0 - 2.9 times baseline creatinine; stage 3, 3.0 times baseline creatinine.
In the comparison of variables among steroid-sensitive and steroid-resistant groups, age > 12 years was associated with steroid resistance while the younger age categories had more steroid-responsive cases (P value = 0.004). In female patients, the presence of hematuria and hypertension were found to be higher in steroid-resistant cases. All patients with secondary causes of nephrotic syndrome were steroid-resistant (Table 3).
| Characteristics of Patients | SSNS | SRNS | P Value |
|---|---|---|---|
| Patients’ age | 0.004 | ||
| 0 - 1 (2)b | 2 (100) | 0 (0) | |
| 2 - 6 (18) | 14 (78) | 4 (22) | |
| 7 - 12 (25) | 18 (72) | 7 (28) | |
| > 12 (18) | 5 (28) | 13 (72) | |
| Patients’ sex | 0.044 | ||
| Male | 35 (69) | 16 (31) | |
| Female | 4 (33) | 8 (67) | |
| Mean cholesterol value | 430 | 294 | 0.270 |
| Mean albumin value | 1.6 | 1.7 | 0.538 |
| Hematuria (42) | 21/39 (54) | 21/24 (88) | 0.060 |
| Type of hematuria | 0.10 | ||
| Gross hematuria | 11 (39) | 17 (61) | |
| Microscopic hematuria | 10 (71) | 4 (29) | |
| Hypertension (31) | 12/39 (31) | 19/24 (79) | 0.00 |
| Acute kidney injury (11) | 6/39 (15) | 5/24 (21) | 0.580 |
| Secondary NS (5) | 0/39 (0) | 5/24 (21) | 0.01 |
aValue are expressed as No. (%).
bAll Patients in this age category were aged > 1 year but less than 2 years.
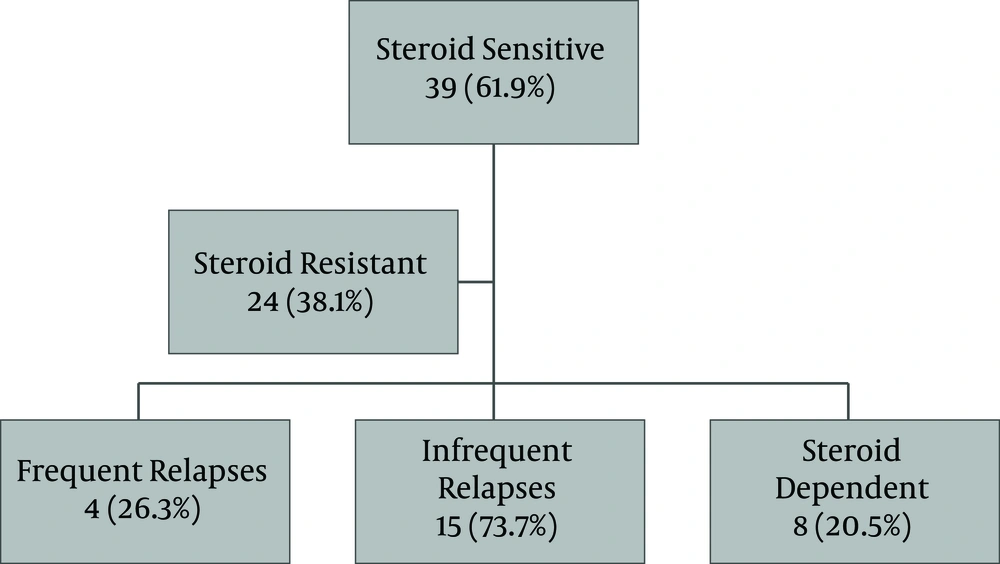
All the patients received prednisolone with 39 cases (61.9%) found to be sensitive, among whom 19 patients (48.7%) had relapses, and 8 cases (20.5%) had steroid dependence. Twenty-four patients (38.1%) were steroid-resistant (Figure 2).
In multivariate logistic regression, age less than 6 years (AOR 16.671, 95% CI: 1.645 - 168.904 P = 0.017) and high cholesterol levels (AOR 1.013, 95% CI: 1.005 - 1.021 P = 0.01) were independent factors affecting steroid response.
5. Discussion
In this study, we reviewed the socio-demographic, clinical, and biochemical characteristics and the steroid response pattern in pediatric patients treated for nephrotic syndrome.
5.1. Socio-Demographic Characteristics
Our study was similar to results from other previous studies in childhood nephrotic syndrome, showing male predominance. The male to female ratio in our study was 4.2:1. A study in Iran showed a male to female ratio of 1.9:1, Saudi (2.1:1), and Kano Nigeria (5.7:1) (9, 23, 24). There is, however, no particular explanation offered for this male preponderance in nephrotic syndrome. Studies have shown that males achieve remission faster, while females tend to respond late with a higher risk of resistance (14, 16). Our study showed that there were more males in the steroid-sensitive category, whereas a greater percentage of the females (67%) were in the steroid-resistant groups (Table 3). This is also similar to studies in Nigeria where boys were more likely to be steroid sensitive than girls (65.2% vs. 34.8%, P = 0.039)) and South-west Iran where the frequency of girls in the steroid-resistant group was higher than boys (16, 25).
Most studies show peak ages of nephrotic syndrome to be less than 5 years. (11, 26). In our study, 25 cases (39.7%) were in the age range of 7 - 12 years with a median age of onset of 3 years. This is similar to studies done in Ibadan, and Kano states, Nigeria (9, 25) but incongruent with a study done in Enugu State, Nigeria, where the peak age was 10 - 15 years (12).
5.2. Clinical and Biochemical Characteristics
Microscopic hematuria can occur in up to 20% of idiopathic nephrotic syndrome, but gross hematuria is rare (3). However, forty-two patients in our study (66.7%) had hematuria, with the majority of cases shown to have gross hematuria (Table 2). In a study in Saudi Arabia, gross hematuria was found in 8% of their patients with nephrotic syndrome, and an Iranian study reported 4.5% (23, 26). On the contrary, a study in Kano, Nigeria, reported that 80% of their patients had microscopic hematuria (9).
In a study done in Louisiana, 28% of the patients had hypertension, which was also found to be a significant predictor of steroid resistance (14). Our study showed that hypertension was present in thirty-one cases (49.2%), with nearly half of patients affected (Table 2). It also accounted for 79% of all the patients with steroid resistant nephrotic syndrome and was found to be a significant predictor of steroid response, as shown in Table 3 (P = 0.000). Other studies reported lower rates of hypertension than that seen in our study (9, 26, 27).
5.3. Response to Steroids
Response to steroid therapy has been reported to be different in various geographical locations and ethnicities (6). In our study, thirty-nine cases achieved remission within 8 weeks of treatment with prednisolone, thus the steroid response was 61.9%. A study done in Nigeria and Iran showed steroid response rates of 63.3% and 66%, respectively (24, 25). A high steroid responsiveness was reported in a study done in South-West Nigeria as 83% for idiopathic nephrotic syndrome, in contrast to other previous African studies that demonstrated lower response rates, including Northern Nigeria (28%) and Ghana (50%). This good response was explained by a younger age of onset and a lack of secondary etiologies (9-11). In our study, the steroid response reported was attributed to a higher age of onset, presence of atypical features, and secondary nephrotic syndrome. This contradicts the results of a study in Enugu State, Nigeria, where the steroid sensitivity was 85.7% with a peak age of onset reported to be 11 - 15 years and secondary NS cases included in the study (12).
Our study showed that older age (> 12 years) was associated with higher steroid resistance rates than the younger age categories with more steroid responsiveness (P value = 0.004) as shown in Table 3. Younger age less than 6 years was also found to be an independent factor affecting steroid responsiveness on multivariate logistic regression (AOR 16.671; 95% CI: 1.645 - 168.904 P = 0.017). This contradicts the results of a study in Ibadan, Nigeria, where the steroid response rates were shown to be similar both in children aged < 6 years and > 6 years (25). Patients with a younger age < 6 years of age are more likely to have a good response to treatment because this is the period where minimal change nephrotic syndrome, the most common histologic type of INS, tends to occur (3).
Cholesterol levels in this study were much higher in the steroid-responsive than steroid-resistant groups (Table 3), and on multivariate logistic regression, high cholesterol values were an independent predictor of steroid responsiveness (AOR 1.013, 95% CI: 1.005 - 1.021 P = 0.01). In the Ibadan study, there were higher mean cholesterol values in the steroid-sensitive as compared to steroid-resistant (410.3 vs. 383.1). However, it was not shown to have statistical significance (P value = 0.554) (25). High cholesterol levels have been shown to be associated with markedly lower albumin levels and patients with relapses in nephrotic syndrome (28, 29). Another study in Nigeria showed that there was a relationship between lower cholesterol levels and steroid response (11). Further studies could be done to ascertain the relationship between higher cholesterol values and steroid response.
5.4. Conclusions
The steroid response in our study was found to be similar to Asian and some African countries. Most patients had features of atypical nephrotic syndrome like hypertension and hematuria with older age at presentation. Independent factors affecting response to treatment with prednisolone in our study include younger age and high cholesterol levels.