1. Background
Urinary tract infections (UTIs) are among the most common bacterial infections occurring during childhood. These infections can induce damages to the bladder, kidneys, and ureters (1). Based on the statistics, UTIs have prevalence rates of 1% and 1% - 3% in boys and girls, respectively (2). These infections have the highest frequency among the patients up to the age of one year in both genders (3). Gram-negative Enterobacteriaceae is the main cause of community-acquired UTI (4, 5). The main etiological agent of this infection is Escherichia coli (E. coli), which accounts for up to 90% of cases, with various frequencies ranging from 47.6% to 85.9 % (5). Although the majority of UTIs are due to E. coli, the diagnosis of the infections caused by organisms other than E. coli is very important, given their different antimicrobial susceptibility patterns (6). Atypical UTI includes seriously ill state, poor urine flow, abdominal or bladder mass, raised serum creatinine levels, septicemia, failure to respond to treatment, and infections with non-E. coli organisms (7). A non-E-coli infection has been suggested as a risk factor for vesicoureteral reflux (VUR). In addition, such kind of infection is associated with an increased risk of UTI recurrence (8-10).
2. Objectives
The identification of predisposing factors for UTI is of paramount importance in the development of novel therapeutic procedures and long-term follow-up. Regarding this, we aimed to compare UTIs due to E-coli and non-E-coli pathogens in the pediatric population based on the presence or absence of predisposing factors of infection. The main predisposing factors considered in the study were urological anomalies [vesicoureteral reflux (VUR) and different types of urinary obstruction] and neurogenic bladder.
3. Methods
A cross-sectional retrospective review was conducted on the recorded information of patients with UTI referred to the Nephrology Clinic of a tertiary academic children hospital from October 2003 to October 2016 (i.e., a 13-year period). The patients with the diagnosis of UTI from birth up to 18 years of age were enrolled if they underwent direct cystography, either voiding cystourethrography (VCUG) or direct radionuclide cystography (DRC) with renal bladder ultrasonography (RBUS). If there was radiologic evidence suggestive of urinary obstruction, diuretic renal scans such as TC99 EC-renal scan or TC99-DTPA scan were recommended. Moderate to severe hydronephrosis [anteroposterior (AP) diameter of renal pelvis ≥ 10 mm ± hydroureter were considered indications for doing diuretic renal scans. Patients with neurologic deficits such as myelodysplasia, spinal trauma, and cerebral palsy underwent urodynamic studies to assess bladder compliance, capacity, and detrusor sphincter synergy, as well as uninhibited bladder contraction in the filling phase. The diagnosis of neurogenic bladder was confirmed by a combination of findings on physical examination, RBUS, VCUG, and urodynamic study.
The urine sampling was performed by means of urinary bags and midstream methods in no toilet- and toilet-trained patients, respectively. The growth of a single organism with a colony forming unit (CFU) of ≥ 105 was considered a positive culture and diagnosed as UTI. In toilet-trained patients with symptomatic UTI (e.g., fever and lower urinary tract symptoms), a growth of ≥ 104 CFU of a single urinary pathogen was regarded as a positive culture. In the samples obtained via urinary bags, the presence of leukocyturia, in addition to positive urine culture, was necessary for the diagnosis of UTI. Leukocyturia was defined as a white blood cell count of (WBC) ≥ 5 (or approximately 25 WBCs per liter) in the high power field (HPF) of urinary sediment in a centrifuged urine sample.
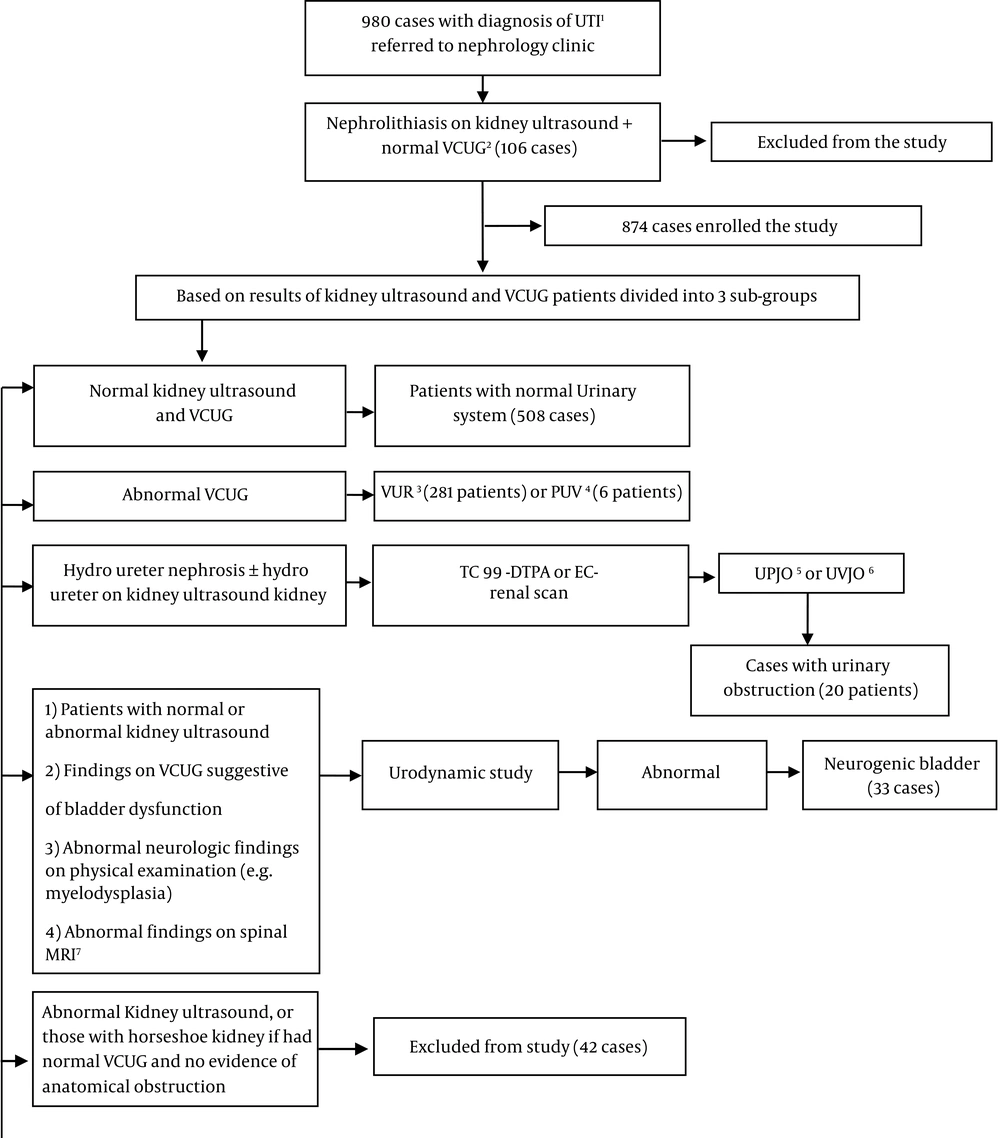
After the implementation of imaging studies, patients with nephrolithiasis but no urological anomalies (n = 106), and those with abnormal kidney ultrasound + normal VCUG with no evidence of anatomical obstruction (n = 46) excluded from the study (total excluded cases = 152 patients). Kidney ultrasound findings in the latter group included mild to moderate hydronephrosis, renal agenesis, or horseshoe kidney. The final enrolled cases (n = 832) were categorized into four groups: patient with apparently normal urinary tract (i.e., normal ultrasound and VCUG findings; n = 518), cases with VUR (n = 281), those with urinary obstruction (n = 20), and cases with neurogenic bladder (n = 33) (Figure 1). Ten cases had a combination of VUR and urinary obstruction.
3.1. Statistical Analysis
Data were analyzed using SPSS software, version 16 (SPSS Institute, Inc., Chicago, IL, USA). Experimental data were presented as mean and standard deviation. All variables showed a normal distribution by using the one-sample Kolmogorov-Smirnov test. Chi-square test was used for data analysis. A P value of less than 0.05 was considered statistically significant.
4. Results
Of 832 enrolled patients, 741 (89.0 6%) and 91(10.94%) cases were girls and boys, respectively. Table 1 presents the demographic characteristics of different groups of patients. Totally 508/832 (61.05%) cases had normal kidney US and VCUG. They defined as patients with apparently normal urinary system, 281/832 (33.77%) subjects were diagnosed as the group with VUR, 33/832 (3.97%) as cases with neurogenic bladder, and 20/832 (2.4%) cases had a urinary obstruction. In 10 (1.2%) subjects, VUR was associated with urinary obstruction.
| Groups of Patients | Age, mo | Gender | E. coli Infections | Non-E. coli Infections | Total | |
|---|---|---|---|---|---|---|
| Girls | Boys | |||||
| Normal urinary tract (508 cases) | 44.05 ± 37.21 | 470 (92.52) | 38 (7.48) | 598 (82.71) | 125 (17.29) | 723 (100) |
| Patients with VUR (281 patients) | 25.38 ± 26.66 | 242 (86.12) | 39 (13.88) | 389 (76.72) | 118(23.27) | 507 (100) |
| Cases with neurogenic bladder (33 cases) | 37.8 ± 43.39 | 23 (69.7) | 10 (30.3) | 55 (66.26) | 28 (33.74) | 83 (100) |
| Subjects with urinary obstruction (20 cases) | 13.8 ± 14.75 | 9 (45) | 11 (55) | 29 (82.85) | 6 (17.15) | 35 (100) |
| Total cases (832 patients)b | 38.48 ± 36.11 | 741 (89.06)c | 91 (10.94)d | 1071 (79.45) | 277 (20.55) | 1348 (100) |
Abbreviation: VUR, vesicoureteral reflux.
aValues are expressed as No. (%) or mean ± SD.
bIn 10 patients, vesicoureteral reflux was associated with urinary obstruction
cThree girls had vesicoureteral reflux in association with urinary obstruction
dSeven boys had vesicoureteral reflux in association with urinary obstruction.
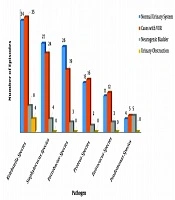
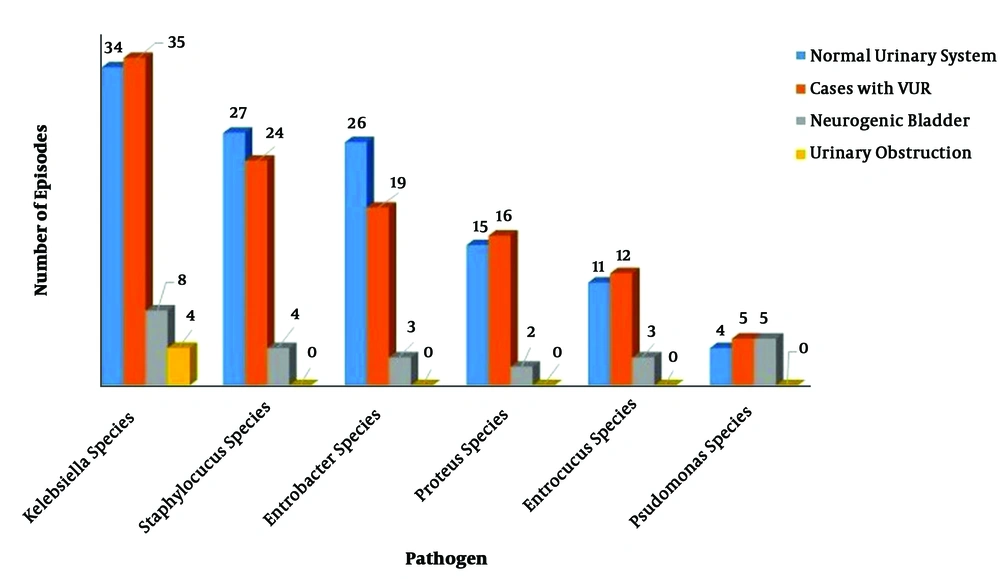
Based on the obtained results of our study, E. coli was responsible for 1071/1348 (79.45%) episodes of infections, while 20.55% (n = 277) of UTI episodes were due to non-E. coli pathogens (Table 1). The most common non-E. coli pathogens in total enrolled cases were Klebsiella (81/277; 29.24%), Staphylococcus (55/277; 19.85%), Enterobacter (48/277; 17.33%), Proteus (33/277; 11.91%), Enterococcus (26/277; 9.4%) and Pseudomonas (14/277; 5.05%) species (Table 2).
| Non-E. coli Pathogens | Normal Urinary System | Cases with VUR | Neurogenic Bladder | Urinary Obstruction | Total |
|---|---|---|---|---|---|
| Kelebsiella | 34 (27.2) | 35 (29.66) | 8 (28.57) | 4 (66.66) | 81 (29.24) |
| Staphylococcus | 27 (21.6) | 24 (20.34) | 4 (14.3) | 0 (0) | 55 (19.86) |
| Enterobacter | 26 (20.8) | 19 (16.1) | 3 (10.71) | 0 (0) | 48 (17.33) |
| Proteus | 15 (12) | 16 (13.56) | 2 (7.14) | 0 (0) | 33 (11.91) |
| Enterococcus | 11 (8.8) | 12 (10.17) | 3 (10.71) | 0 (0) | 26 (9.39) |
| Pseudomonas | 4 (3.2) | 5 (4.23) | 5 (17.86) | 0 (0) | 14 (5.05) |
| Citerobacter | 6 (4.8) | 1 (0.84) | 1 (3.57) | 1 (16.67) | 9 (3.25) |
| Group B Streptococcus | 1 (0.8) | 4 (3.4) | 1 (3.57) | 0 (0) | 6 (2.17) |
| Group A Streptococcus | 0 (0) | 2 (1.7) | 0 (0) | 0 (0) | 2 (0.72) |
| Acinetobacter | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (0.36) |
| Serattia | 0 (0) | 0 (0) | 1 (3.57) | 0 (0) | 1 (0.36) |
| Morganella morganii | 0 (0) | 0 (0) | 0 (0) | 1 (16.67) | 1 (0.36) |
| Total | 125 (45.13) | 118 (42.6) | 28 (10.1) | 6 (2.17) | 277 (100) |
Abbreviation: VUR, vesicoureteral reflux.
aValues are expressed as No. (%).
Non-E. coli pathogens were responsible for 125, 118, 28, and 6 episodes of infection in cases with apparently normal urinary tract, patients with VUR, case with neurogenic bladder, and those with urinary obstruction, respectively (P = 0.003). Based on the obtained results, non-E. coli infections were significantly more prevalent in cases with neurogenic bladder compared to three other groups of patients. Pseudomonas species were responsible for 3.2% (4/125), 4.23% (5/118), 17.85% (5/28), and no episode of infection (0%) in cases with apparently normal urinary tract, patients with VUR, cases with neurogenic bladder, and those with urinary obstruction, respectively (P = 0.004). It means that infection with Pseudomonas species was significantly more prevalent in patients with neurogenic bladder compared to other groups. Klebsiella was the most common non-E. coli pathogens in all groups of the patients (Figure 2).
5. Discussion
The results of the current study revealed that E. coli was the cause of UTI in the majority of the cases. Moreover, it was the most frequent etiologic agent in all four groups of patients. Most of the patients were female and had febrile UTI. We found a significantly higher frequency of non-E. coli infections in cases with neurogenic bladder vs. those with apparently normal urinary tract and patients with urological anomalies (VUR and urinary obstruction). Shaikh et al. (6) reported that Hispanic children and cases with moderate to severe VUR (VUR grade 3 - 5) are more prone to develop UTI caused by non-E. coli organisms.
The present study was targeted toward the assessment of the rate of UTI caused by E. coli and non-E. coli pathogens among Iranian children. Our results revealed a higher prevalence of UTI among females than males. Nonetheless, this rate has been reported to be higher in males than in females in other studies (3, 11). This discrepancy may be due to the use of various sampling methods. In this regard, in a study performed by Spahiu and Hasbahta (3), 63.88% of the infections were reported to be caused by E. coli pathogens. Furthermore, Halerstein (11) and Shaikh et al. (6) reported this rate as 75%. In the present study, E. coli was the cause of UTI in 79.5 % of the infections. Friedman et al. (12) found a higher association of non-E. coli disease with urinary tract anomalies (77%), including VUR (50%) and UPJO (9%).
Despite different studies on pediatric UTIs, our knowledge about non-E. coli pathogens is incomplete (1). A better understanding of the non-E. coli pathogens accounting for the development of UTI can contribute to the prevention and management of this disease. Based on the literature, a total of 10 -20% of UTI episodes are due to non-E. coli pathogens. Klebsiella, Enterobacter, Pseudomonas, and Staphylococcus species are the most common non-E. coli pathogens accounted for pediatric UTIs (3). In the current study, approximately 20% of the infections were due to non-E. coli pathogens, 29.24% of which were due to Klebsiella. Klebsiella accounted for 81/1348 (6%) of total UTI episodes. Compared to the study by Spahiu and Hasbahta (3), the prevalence rate of UTIs developed by Klebsiella was about four-fold of our series (23.06% Vs. 6%, respectively). They found Proteus mirabilis, Citrobacter, and Staphylococcus saprophyticus as the second to fifth most common non-E. coli uropathogens in their cases. In our series, Proteus and Citrobacter were responsible for 2.45% and 0.66% of total UTI episodes, respectively. Staphylococcus species were accounted for 4.08% of total episodes of UTIs. In the current study, the second to fifth most common non-E. coli uropathogens were Staphylococcus, Enterobacter, and Proteus species, respectively.
In a study carried out by Friedman et al. (12), in terms of clinical and laboratory characteristics of UTI, there was a significant difference between the infants and children. They reported that patients with non-E. coli UTIs younger, have milder clinical signs, and need a longer hospital stay in comparison to those with E. coli-related UTIs. Moreover, they showed a higher rate of urinary tract anomalies in patients with non-E. coli UTIs compared to those affected by E. coli species. In the current study, E. coli and non-E. coli pathogens accounted for 77% and 23 % of infections in cases with VUR, and 82% and 18% of UTIs in a patient with urinary obstruction.
The results obtained by Honkinen et al. (13) showed a stronger association between non-E. coli UTIs (i.e., Klebsiella or Enterococcus UTIs) and VUR than between E. coli UTIs and VUR, in contrast to this study, we did not find a significant association between non-E. coli infections and VUR. Based on a study carried out by Shaikh et al. (6), the UTIs are caused by non-E. coli pathogens are more observed among children with grade III or IV VUR and those without fever. They showed that the rate of high-grade VUR in children with non-E. coli UTIs was twice the rate of the UTIs caused by E. coli (6). This result has also been confirmed in previous studies (13, 14). Therefore, inconsistent with the findings of other studies, we found that non-E. coli UTIs cannot be suggested as a predictive factor for the diagnosis of anatomic anomalies. Nonetheless, our results indicated a significantly higher prevalence of non-E. coli UTIs in patients with neurogenic bladder dysfunction.
The value of non-E. coli virulence factors in patients with neurogenic bladder has not been established yet. To the best of our knowledge, no sequence type or phylogenetic group of non-E. coli has been observed in the urine culture of the patients suffering from the neurogenic bladder. In addition, there are no data demonstrating how they are genetically related to other pathogens (6).
In a study, 25 children with neurogenic bladder receiving clean intermittent characterization were assessed for UTI. During 24 weeks, weekly urine samples were obtained through a urinary catheter. In 56% of cases, the urine culture was positive. The most prevalent organisms were E. coli, Klebsiella, Coagulase-negative staphylococci, Corynebacterium, and Enterococcus species, respectively. In our series, the most common organisms among patients with diagnosis of neurogenic bladder were E. coli, Klebsiella, Pseudomonas, Staphylococcus, Enterobacter, and Enterococcus species, respectively.
A relationship has been reported between the higher rate of E. coli UTIs in patients with a normal urinary tract and higher virulence of E. coli in comparison to non-E. coli strains. Abnormal urinary tract is more susceptible to infection with the less virulent non-E. coli bacteria (15). Shaikh et al. (6) found a high number of urinary tract anomalies in patients with non-E. coli UTI necessitates the administration of prolonged antibiotic therapy and longer hospital stay. They found antibiotic treatment before hospitalization as a main risk factor for non-E. coli UTIs. Non-E. coli species are more likely to be resistant to the first-generation cephalosporins and nitrofurantoin (16).
Honkinen et al. (13) reported VUR in 1/3 of children affected by E. coli UTIs. They also found a double rate of VUR in patients whose first episodes of UTIs were due to Klebsiella or Enterococcus (non-E. coli pathogens). In our series, E. coli was the pathogen responsible for first episodes of UTIs in 326/551 (59.16%) VUR– and 233/281 (82.9%) VUR+ patients. Klebsiella accounted for 48/832(5.77%) of the first episodes of UTIs, including 25/281(8.9%) patients with VUR. In VUR– cases, 23/551 (4.17%) episodes of the first UTIs were due to Klebsiella. It indicated that Klebsiella infections at the first UTIs were 2.13 times more common in patients with Vs. those without VUR. In the current study, Enterococcus was responsible for 12/832 (1.44%) of the first episodes of UTI, including 7/281 (2.5%) episodes in patients with and 5/551(0.8%) episodes in those without VUR. Enterococcus was not responsible for the first episodes of UTI in any patient with urinary obstruction or neurogenic bladder.
5.1. Conclusions
The findings of the present study indicate the patients with neurogenic bladder dysfunction are more prone to develop not only non-E. coli UTIs but also Pseudomonas infections than those with normal urinary tract or urological anomalies. We demonstrated that neither presence of VUR nor its severity (severe vs. mild to moderate VUR) are not significantly associate with non-E. coli UTIs. Doing similar studies in patients with voiding dysfunction ± VUR seems to be attractive. Voiding dysfunction or non-neurogenic bladder, an entity mimics clinical manifestation and radiologic findings of neurogenic bladder, is common among the pediatric population affected by UTI. Such evaluation can determine whether, similar to the patients with neurogenic bladder, those with voiding dysfunction are more prone to develop non-E. coli UTIs or not?