1. Background
Mycophenolate mofetil (MMF) or mycophenolate sodium (MPS), an ester prodrug of mycophenolic acid, is an immunosuppressant suggested to be used together with a calcineurin inhibitor and corticosteroid for the renal allograft rejection prevention (1-3). Mycophenolic acid (MPA) is transformed into an inactive phenolic glucuronide (MPAG) that either undergoes enterohepatic cycling or is excreted via the urine (4).
The concentration of MPA in adult kidney transplant recipients in the area under the curve (AUC) is about 10 fold higher than the given dose (5, 6). The risk of acute rejection could be predicted based on MPA-AUC values (7). A large central study that determined the relationship between the MPA-AUC and rejection biopsy evidence showed that the rejection rates had a decreasing trend with mean AUC values between 30 - 60 mg.h/L (8). About 97% - 98% of MPA in the body is in protein binding form; however, the small part in free form is pharmacologically active (9).
There are many highly specific methods to quantify the MPA concentration for TDM such as enzyme immunoassay, high-performance liquid chromatography (HPLC). This study aimed to determine the MPA concentration on Vietnamese renal transplant recipients.
2. Objectives
In this regard, the enzyme immunoassay method was used to determine the MPA level with the previously reported satisfactory analytical performance.
3. Methods
3.1. Study Protocol
This cross-sectional study was performed in the transplantation ward of Viet Duc Hospital. Patients of a consecutive series were included on day 3, day 10, and month 6 after kidney transplantation. All patients received MPA (1 g twice a day, ranged from 0.5 to 1 g twice a day at month 6 orally by Cellcept or Myfortic) as a part of the immunosuppression protocol.
3.2. Blood Sampling and Drug Assays
Blood samples were collected into tubes containing EDTA before the patients received MPA (time 0 min) and at 1, 2, 3, and 6 hours after receiving 1 g MPA per day orally. The plasma was collected from centrifuged blood, then stored at -22°C until analyzed.
The MPA level were analyzed in the Inkido chemical system by the CEDIATM mycophenolic acid immunoassay kit (Lot 100276, Thermo Fisher). The assay is based on the activity of the β-galactosidase enzyme. This enzyme catalyzes the cleavage of MPA to generate a color change that can be measured by spectrophotometry. The MPA level was calculated based on the calibrator (Lot 100277). The least detectable dose is 0.2 µg/mL. The MPA-AUC was calculated by the linear trapezoidal rule at five sampling time points (predose, 1, 2, 3, and 6 hours).
3.3. Statistical Analyses
The MPA-AUC0-12 h were calculated by using the linear trapezoidal rule plasma concentration drawn 12 h after drug administration. Categorical data are expressed as a percentage. Continuous variables are presented as means ± standard deviation (SD). Statistical significance was defined by P < 0.05. Statistical analyses were performed with SPSS 16.0 software.
4. Results
4.1. Study Population Characteristics
Thirty-five kidney transplant recipients (22 male and 13 women) were included in the study on day 3, day 10, and month 6. Patient demographics are presented in table 1. Immunosuppressive therapy used for these patients was a combination of cyclosporine (CsA) (4 patients) or tacrolimus (31 patients), with prednisolone and MPA. The patients started with the 1 g twice a day MMF dose or 720 mg twice a day MPS, the real MMF doses ranged from 0.5 - 1 g twice a day or MPS doses ranged from 360 - 720 mg twice a day on month 6. At that time, there was a patient who changed drug and a patient who refused the study.
| Range | |
|---|---|
| Number of patients (male/female), n | 35 (22/13) |
| Age, y | 38.17 ± 12 |
| Body weight, kg | 54.5 ± 10 |
| Donor type (living/cadaveric) | 29/6 |
| Using of CNI (Tac/CsA) | 31/4 |
| Using of MMF (Cellcept/Myfortic) | 24/11 |
Abbreviations: CNI, calcineurin inhibitor; CsA, cyclosporine; Tac, tacrolimus.
4.2. The MPA-AUC0-12 of Kidney Transplant Recipients
Plasma MPA total levels ranged from 0.1 to 8.8 mg/L, and the estimated MPA-AUC0-12 h values were 10.73 to 129.05 mg.h/L. The comparison of active values is summarized in Table 2. The MPA and MPA-AUC levels were lower in the first few days than month 6 after kidney transplantation (P < 0.01). The MPA-AUC values were 30 - 60 mg.h/L in 18 patients (51.4%), 23 patients (65.7%), 17 patients (51.5%) at day 3, day 10, and month 6. The MPA C0 values were 1.5 - 2.5 mg/L in 15 patients (42.9%), 14 patients (40%), 10 patients (30.3%) on day 3, day 10, and month 6. The pharmacokinetics parameters of MPA of patients showed that the Cmax varied from time 1 to time 6, only 2 cases at 6 hours and the other was 1, 2, or 3 hours, and 8 patients did not change on day 3, day 10, and month 6.
| Concentrate | Day 3 | Day 10 | Month 6 |
|---|---|---|---|
| C0, mg/L | 2.32 ± 1.47 | 1.58 ± 1.39 | 2.29 ± 1.4 |
| AUC0-12h, mg.h/L | 50.1 ± 20.4 | 41.9 ± 14.5 | 60.3 ± 25.9 |
5. Discussion
In the current study, the immunosuppression therapy was the combination of MMF, oral prednisolone, and calcineurin inhibitor (cyclosporine or tacrolimus were monitored). The MMF is proven to be an effective drug in immunosuppression therapy to prevent early acute rejection (10). These studies showed the outstanding results of MMF (with 2 g per day) compared to azathioprine regarding better acute rejection risks and safety when combined with calcineurin inhibitor (11). Although 2 g per day has been suggested as the optimal dose, a number of side effects may be observed, such as hematologic disorders, gastrointestinal irritations, especially with patients taking Cellcept. If these side effects progressed severely, the MPA dose would have to be reduced, which means the risk of rejection might increase. A new method for overcoming these obstacles was changing Cellcept to enteric-coated mycophenolate sodium (Myfortic). With this new version of the MPA drug, the drug tolerance was better, and the dose could also be increased higher (12).
Many researches that used the combination of calcineurin inhibitor and MPA showed that the pharmacokinetics of MPA was related to acute rejection risk and side effects when the daily dose was fixed with 2 g. The lower MPA-AUC0-12 h was, the higher risk of rejection was. Patients who experienced MMF-related side effects often had a reduction dose that meant they would have low AUC and a high risk of rejection (8-10). Patients with low MPA-AUC had a high risk of acute rejection than those with high MPA-AUC, and the AUC had a higher value in prediction than C0 (13). The Randomized Concentration Controlled study on MMF had proven the concern between the MPA-C0, MPA-AUC, and the incidence of acute rejection but with side effects in both technical (8) and clinical (9) aspects. Several studies suggested an appropriate MPA-AUC in renal transplant recipients for decreasing the risk of acute rejection in the range of 30 to 60 mg.h/L (8, 14, 15). The AUC on day 3, day 10, and month 6 was 18 (51.4%), 23 (65.7%), 17 (51.5%), respectively, that were within the therapeutic range of 30 - 60 mg.h/L (Table 3). The numbers of patients who had the optimal AUC were different because of different timelines after transplantation and lower dose at month 6. Several studies in kidney transplant recipients have demonstrated that in the first few weeks after transplant, the mean total MPA-AUC was 30-50% lower than at 2 to 6 months after transplant (15, 16). In the present study, the median MPA-AUC increased from 50.1 mg.h/L and 41.9 mg.h/L at day 3 and day 10 to 60.3 mg.h/L at month 6 after transplantation.
| C0, mg/L | AUC0-12, mg.h/L, No. (%) | Total, No. (%) | ||
|---|---|---|---|---|
| < 30 | 30 - 60 | > 60 | ||
| Day 3 | ||||
| < 1.5 | 5 (14.3) | 4 (11.4) | 1 (2.9) | 10 (28.6) |
| 1.5 - 2.5 | 0 (0.0) | 11 (31.4) | 4 (11.4) | 15 (42.9) |
| > 2.5 | 0 (0.0) | 3 (8.6) | 7 (20.0) | 10 (28.6) |
| Total | 5 (14.3) | 18 (51.4) | 12 (34.3) | 35 (100) |
| P-values | 0.000 | |||
| Day 10 | ||||
| < 1.5 | 7 (20.0) | 12 (34.3) | 1 (2.9) | 20 (57.1) |
| 1.5 - 2.5 | 2 (5.7) | 10 (28.6) | 2 (5.7) | 14 (40.0) |
| > 2.5 | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (2.9) |
| Total | 9 (25.7) | 23 (65.7) | 3 (8.6) | 35 (100) |
| P-values | 0.574 | |||
| Month 6 | ||||
| < 1.5 | 2 (6.1) | 7 (21.2) | 2 (6.1) | 11 (33.3) |
| 1.5 - 2.5 | 0 (0.0) | 7 (21.2) | 3 (9.1) | 10 (30.3) |
| > 2.5 | 0 (0.0) | 3 (9.1) | 9 (27.3) | 12 (36.4) |
| Total | 2 (6.1) | 17 (51.5) | 14 (42.4) | 33 (100) |
| P-values | 0.021 | |||
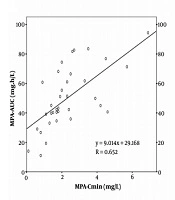
The MPA-AUC in the early periods was lower than later periods after transplantation because of drug interaction between MMF and cyclosporine. Otherwise, poor gastrointestinal conditions might affect MMF absorption. However, MPA metabolism was increased because of the high dose of glucocorticoid, which was used together with MPA, might reduce the UDP-GT activity (17). The concentration of MPA-AUC correlated with the MPA C0 (Figure 1). The C0 on day 3, day 10, month 6 were 15 (42.9%), 14 (40%), 10 (30.3%) in the study, respectively, that were within the therapeutic range (Table 3). These results were similar to a retrospective study in 48 renal post-transplantation patients, MPA level in patients with rejection was significantly lower than those without rejection (1.55 ± 0.48 vs. 2.11 ± 0.62 mg/L) (18).
Although MPA-AUC from 0 to 12 hours is the best predictor of acute graft rejection, the ability to collect many sampling time points for MPA-AUC determination is infeasible in clinical practice. The MPA-AUC in this study was calculated from the concentration values of MPA at 5 sampling time points., there was a peak (Cmax), and 2 hours had the highest concentration with 37.1% and 40% (days 3 and 10) and 57.6% (month 6) (Table 4). In Thai kidney transplant recipients, MPA concentration at 2 hours had the highest correlation with MPA-AUC (r = 0.622); the time of the study was 4 months (19). In the study of Honarbakhsh et al. (20), the recipients showed one peak, two peaks, or three peaks, and the time of the study was days 9 or 10. These differences depended on many factors including race, immunosuppression protocol, time from transplant, or MMF dosage. The main limitation of the study was the deficiency of histopathological information that reflected the rejection status. That was important evidence for the effectiveness of immunosuppressive therapy. Moreover, the number of patients was small, and the following time was not too far, only six months.
| Peak | Day 3, No. (%) | Day 10, No. (%) | Month 6, No. (%) |
|---|---|---|---|
| C1 | 12 (34.3) | 11 (31.4) | 19 (57.6) |
| C2 | 13 (37.1) | 14 (40) | 7 (21.2) |
| C3 | 8 (22.9) | 8 (22.9) | 8 (24.2) |
| C6 | 2 (5.7) | 2 (5.7) | 0 (0.0) |
| Total | 35 (100) | 35 (100) | 33 (100) |