1. Background
Hemodialysis patients experience a hefty burden of physical and emotional symptoms (1). The average prevalence of depression in hemodialysis patients is approximately 20% to 30% (2). The prevalence of depression in patients on hemodialysis is three times higher than that in the general population. These patients are prone to depression for various biological, psychological, and social reasons (3). The increased levels of cytokines and changes in neurotransmitter levels will influence the formation of depression among hemodialysis patients. According to various studies, inflammatory factors such as interleukin-1, interleukin-2, and tumor necrosis factor-alpha (TNF-α) are common among these patients (4, 5).
Due to the severity of the issue, effective depression therapies are required in routine dialysis care (6). Influential factors include pharmacological and non-pharmacological therapies such as psychotherapy (interpersonal psychotherapy, cognitive and behavior therapy), electroconvulsive therapy (ECT), or a combination of them, which bring negative feedback and dissatisfaction (3, 7). These treatment options, especially the administration of antidepressants, have been associated with little to no evidence of efficacy and safety. As a result, patients are often excluded from major clinical trials due to safety concerns. The number of studies carried out on subjects belonging to a small population cannot be generalized to the whole community (8). Most antidepressants are protein-bound and will be metabolized in the liver; therefore, they are not eliminated by dialysis. However, the relative activity and the excretion of these metabolites in patients with chronic kidney disease (CKD) are often unclear (9).
Cool dialysate is an intervention that can improve the quality of life of patients, boost sleep quality, reduce fatigue, enhance the dialysis quality, alleviate itching, and improve restless legs syndrome (RLS) (10, 11). Cold dialysis reduces some physical problems, which are significant causes of depression in hemodialysis patients. Therefore, this intervention can reduce depression severity among the patients (10). Among multiple studies on the application of cool dialysate, the work by Ghanbarabadi et al. (12), Soleimani et al. (13), and Parker et al. (14) led to the increased quality of sleep in hemodialysis patients. According to Sekercioglu et al.’s study (15), conducted on sleep quality, depression, and quality of life in patients with chronic renal failure, it can be concluded that cool dialysate may affect depression.
Fatigue is one of the most prevalent and distressing symptoms that affect individuals’ quality of life. Due to the high frequency of fatigue in patients with chronic renal failure (70% - 97%) and its association with depression, it can be said that daily hemodialysis reduces the symptoms of depression and fatigue, which is in line with the findings of some studies (16-21). According to studies, cool dialysate is an appropriate method to increase the tolerance of dialysis and reduce fatigue, thereby improving depression (22). Furthermore, cool dialysate will boost the patients’ levels of energy and general health with no adverse effect on dialysis adequacy (19).
2. Objectives
In the light of earlier mentioned criteria, this study aimed to evaluate the effect of cool dialysate on depression in patients with chronic renal failure treated with hemodialysis in Sabzevar.
3. Methods
This is a two-group, triple-blind, randomized clinical parallel trial performed with a before-after design (Iranian Registry of Clinical Trials code: IRCT20200215046495N1). Sampling started on April 20, 2020, and finished on July 21, 2020, at two hemodialysis centers of Vasei Hospital and Kashefi Center. The sample size was determined using G*Power statistical software according to the information obtained from the first 10 patients in each group. The confidence level, test power, and drop-out rate were calculated as 95%, 80%, and 20%, respectively.
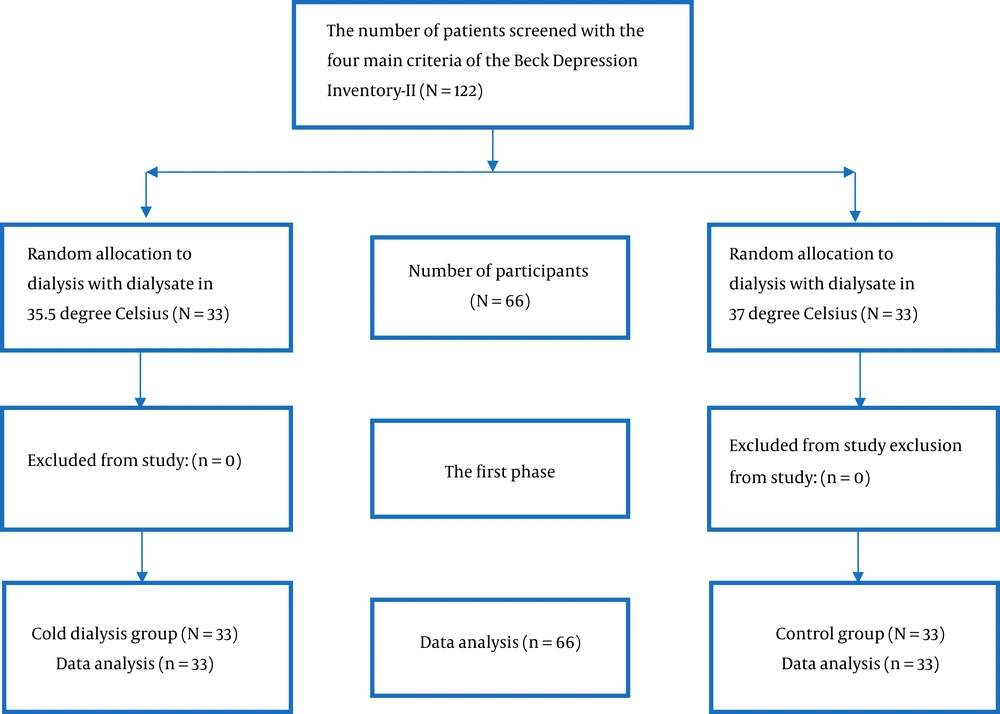
After screening by a depression questionnaire, 122 patients were selected from the two centers. According to the inclusion and exclusion criteria, 66 individuals were randomly allocated by the permuted-block method to a control group (33 patients treated with 37°C cool dialysate) or an intervention group (33 patients treated with 35.5°C cool dialysate). The inclusion criteria included informed consent to participate in the study, age of older than 18 years for both genders, being ultimately conscious, and having acceptable speech and listening ability to answer the questions. The exclusion criteria included patients administrated with antidepressants, underactive thyroid (hypothyroidism), and hemoglobin count of less than 8 mg/dL (Figure 1).
Data were acquired by a demographic questionnaire and the Beck Depression inventory-II (BDI-II). The validity of BDI-II was determined in the study by Taheri Tanjani et al. (as cited by Hamidi) with an internal correlation (IC) coefficient of 0.81 (20). The reliability of the BDI-II questionnaire were measured by Cronbach’s alpha method of as 83%.
The BDI-II questionnaire consisted of 21 items composed of four sentences. The participants were supposed to encircle the one that expressed their feelings and behaviors. Each item was scored from zero to three, so the participants could achieve a score ranging from 0 to 63. Depression severity was classified into four categories based on the scores: zero to 13 (no or minimal depression), 14 to 19 (mild depression), 20 to 28 (moderate depression), and 29 to 63 (severe depression).
The hemodialysis was performed with a filter, and the device had been calibrated for temperature regulation before the process. Data were collected in three shifts in the morning, afternoon, and evening from the hemodialysis centers. Initially, BDI-II was filled out during hemodialysis before the intervention when the patient was in a calm state by the research assistant for those who met the inclusion criteria.
The temperatures of hemodialysis for the standard and cool dialysate patients were 37°C and 35.5°C, respectively. To manage the research blindly, the data were collected by an assistant. The authors were unaware of both the type of intervention and the allocated groups. Patients were also unaware of the group they were assigned to. This was achieved by covering the screen of the device with a 2 × 1 cm paper so that the participants did not find out which study groups they have been placed in.
The BDI-II questionnaire was filled out before the study. One month after the intervention, to measure depression in both intervention (cool dialysate) and control groups (standard dialysate), depression was evaluated in both groups two weeks later to check the persistency of the intervention. Participants were asked to answer all the information accurately and honestly. The researcher was blinded by assigning two codes of A and B to the groups. He/she was unaware of the allocation method of patients during the analysis.
After sampling and data collection, the forms were coded, and their data were imported into SPSS version 25 software to be analyzed. The Shapiro-Wilk test was used to examine the normal distribution of quantitative data. The inferential data were analyzed by the repeated-measures ANOVA (RM-ANOVA) with a significance level of 0.05.
This study was approved and registered with the code of IR. MEDSAB. REC. 1398. 116 in the Ethics Committee of Sabzevar University of Medical Sciences. The confidentiality of patients’ information was monitored throughout the study. All the subjects gave their informed consent. They were aware that participating in this study was completely voluntary and free from any obligation by the treating physician, nursing staff, the researcher, and the research assistant.
4. Results
Table 1 shows the demographic characteristics and medical records of patients.
| Statistical Index | Group | Statistical Results | |||
|---|---|---|---|---|---|
| Control (N = 33) | Intervention (N = 33) | Test Result | Degrees of Freedom | P-Value | |
| Age, y | 58.67 ± 14.44 | 56.33 ± 14.84 | t-test; t = 0.64 | 64 | 0.52 |
| Duration of chronic renal failure, mo | 58.30 ± 50.15 | 90.12 ± 95.04 | Mann-Whitney U; Z = 0 -0.71 | 0.47 | |
| Duration of hemodialysis treatment, mo | 28.00 ± 18.67 | 43.61 ± 39.30 | Mann-Whitney U; Z = 0 - 0.97 | 0.32 | |
| Adequacy of dialysis, KT/V before intervention | 1.15 ± 0.18 | 1.30 ± 0.27 | Mann-Whitney U; Z = 0 - 0.47 | 0.01 | |
| Hemoglobin level, mg/dL | 12.18 ± 1.89 | 12.09 ± 2.12 | t-test; t = 0.17 | 64 | 0.17 |
| Gender | Chi-square = 0.28 | 1 | 0.59 | ||
| Female | 11 (33.3) | 9 (27.3) | |||
| Male | 22 (66.7) | 24 (72.7) | |||
| Education level | Fisher’s Exact test | 0.28 | |||
| Illiterate | 9 (27.3) | 9 (27.3) | |||
| Basic literacy | 4 (12.1) | 2 (6.1) | |||
| Primary | 8 (24.2) | 8 (24.2) | |||
| Secondary | 3 (9.1) | 8 (24.2) | |||
| High school | 2 (6.1) | 4 (12.1) | |||
| Academic | 7 (21.2) | 2 (6.1) | |||
| Occupational status | Fisher’s exact test | 0.99 | |||
| Employed | 2 (6.1) | 2 (6.1) | |||
| Self-employed | 14 (42.4) | 12 (36.4) | |||
| Student | 1 (3) | 1 (3) | |||
| Housekeeper | 8 (24.2) | 9 (27.3) | |||
| Unemployed | 3 (9.1) | 3 (9.1) | |||
| Retired | 5 (15.2) | 6 (18.1) | |||
| Marital status | Fisher’s exact test | 0.34 | |||
| Married | 24 (72.7) | 28 (84.8) | |||
| Single | 4 (12.1) | 2 (6.1) | |||
| Widowed | 5 (15.2) | 2 (6.1) | |||
| Divorced | 0 (0) | 1 (3) | |||
| Income status | Fisher’s exact test | 0.99 | |||
| Adequate | 12 (36.4) | 11 (33.3) | |||
| Inadequate | 20 (60.6) | 20 (60.6) | |||
| Satisfactory | 1 (3) | 2 (6.1) | |||
| Place of residence | Chi-square = 0.08 | 1 | 0.76 | ||
| Urban | 25 (75.8) | 26 (78.8) | |||
| Rural | 8 (24.2) | 7 (21.2) | |||
| Living condition | Fisher’s exact test | 0.92 | |||
| With spouse | 7 (21.2) | 7 (21.2) | |||
| With children | 1 (3) | 1 (3) | |||
| With spouse and children | 17 (51.6) | 20 (60.6) | |||
| Alone | 4 (12.1) | 2 (6.1) | |||
| Other | 4 (12.1) | 3 (9.1) | |||
| History of kidney transplantation | Fisher’s exact test | 0.35 | |||
| Positive | 1 (3) | 4 (12.1) | |||
| Negative | 32 (97) | 29 (87.9) | |||
| Arteries pathway | Fisher’s exact test | 0.43 | |||
| Permanent catheters (Shaldon) | 5 (15.1) | 2 (6.1) | |||
| Fistula | 26 (78.8) | 27 (81.8) | |||
| Artificial veins | 2 (6.1) | 4 (12.1) | |||
| History of certain diseases | Fisher’s exact test | 0.30 | |||
| Blood pressure | 9 (27.3) | 11 (33.2) | |||
| Diabetes | 1 (3) | 0 (0) | |||
| Heart disease | 3 (9.1) | 2 (6.1) | |||
| Blood pressure and diabetes | 14 (42.4) | 9 (27.3) | |||
| Blood pressure and heart disease | 2 (6.1) | 2 (6.1) | |||
| Blood pressure, diabetes, and heart disease | 3 (9.1) | 2 (6.1) | |||
| Polycystic kidney | 1 (3) | 1 (3) | |||
| Other | 0 (0) | 5 (15.2) | |||
| No disease | 0 (0) | 1 (3) | |||
| Taking antipsychotics | Chi-square = 0.00 | 1 | 0.99 | ||
| Positive | 9 (27.3) | 9 (27.3) | |||
| Negative | 24 (72.7) | 24 (72.7) | |||
| Type of antipsychotics | Fisher’s exact test | 0.23 | |||
| Alprazolam | 4 (12.2) | 8 (24.3) | |||
| Clonazepam | 1 (3) | 1 (3) | |||
| Sodium valproate (Depakene) | 3 (9.1) | 0 (0) | |||
| Level and Depakene | 1 (3) | 0 (0) | |||
| None | 24 (72.7) | 24 (72.7) | |||
aValues are expressed as mean ± SD or No. (%).
There was no difference between the groups in terms of demographic and disease information using t-test, Mann-Whitney U-test, chi-square test, and Fisher’s exact test. Before the intervention, the mean and standard deviation of depression in the control and intervention groups were 26.15 ± 1.46 and 25.56 ± 1.28, respectively. The independent t-test showed no significant difference between the two groups (t-value = 0.23, P-value = 0.81). One month after the intervention, the mean scores of depression in the control and intervention groups were 22.24 ± 2.00 and 22.41 ± 1.65, respectively. The independent t-test showed no significant difference between the two groups (t-value = -0.6, P-value > 0.95) (Table 2).
| Group | t-test Results (Intergroup Comparison) | ||||
|---|---|---|---|---|---|
| Control, Mean ± SD | Intervention, Mean ± SD | t | df | P-Value | |
| Before intervention | 26.15 ± 1.46 | 25.56 ± 1.28 | 0.23 | 64 | 0.81 |
| One month after intervention | 22.24 ± 2.00 | 22.41 ± 1.65 | -0.06 | 63 | 0.95 |
| Two weeks after intervention | 22.71 ± 1.92 | 24.28 ± 1.96 | -0.57 | 61 | 0.57 |
| Paired t-test results (intragroup comparison) (t, df, and P-value) | |||||
| Before intervention and two weeks after intervention | (3.68, 30, 0.00) | (0.78, 31, 0.43) | - | - | |
| After intervention and two weeks after intervention | (-50, 30, 0.61) | (-1.06, 31, 0.29) | - | - | |
The results of RM-ANOVA for depression indicated that time had a statistically significant effect on the depression rate (P-value < 0.05). There was no statistically significant effect for the interaction of time and groups (P-value > 0.05). Furthermore, the mean of depression was not statistically significantly different between the studied groups (P-value > 0.05), indicating that the severity of depression was not significantly different (Table 3).
| Source | Statistical Index | ||||
|---|---|---|---|---|---|
| Sums of Squares Type III | Degree of Freedom | Average Root | Frequency | P-Value | |
| Time | 482.53 | 2 | 241.26 | 7.04 | 0.00 |
| Group*time | 54.15 | 2 | 27.07 | 0.79 | 0.45 |
| Group | 3.62 | 1 | 3.62 | 0.01 | 0.89 |
5. Discussion
Based on the findings, it was indicated that cool dialysate did not reduce the depression level in hemodialysis patients. The study by Ahmadi et al. compared the impact of cool dialysate with standard dialysate in terms of detoxification. It revealed that cool dialysate was effective in preventing hypotension (23). Hypotension is a common complication of dialysis that causes the premature discontinuation of dialysis and severely reduces dialysis adequacy. The reduced adequacy of dialysis will give rise to severe physical complications. The present study investigated the impact of cool dialysate on reducing the physical problems of hemodialysis patients, which could alleviate the severity of depression.
Sajadi et al. (22) examined the positive effect of cool dialysate on reducing fatigue (found out to be 31.3%) as one of the physical problems of hemodialysis patients. However, after the intervention, the level of depression did not decrease due to various physical, economic, spiritual, and psychological factors among patients. Furthermore, Kashani et al. (21) studied the alleviating impact of cool dialysate on RLS as disturbing physical factors in hemodialysis patients. Similarly, Ghanbarabadi et al. (12), Soleimani et al. (13), and Parker et al. (14) investigated the effect of cool dialysate on sleep quality. Rad et al. (24) and Ham et al. (25) examined the effect of cool dialysate on itching and exhaustion and found that it improved these complications after the intervention. Researchers assume that cool dialysate could reduce depression by minimizing its side effects. Besides, the application of cool dialysate improved life quality and created high energy levels and liveliness in patients, which, in turn, gave rise to reduced mortality, hospitalization, and dependence on medication.
But in this study, cold dialysis had no effect on depression. Perhaps the reason for this lack of effect is causes other than physical factors. Factors such as mental health problems, anxiety, impaired body image and self-concept, fear of death, treatment limitations, job disability, feelings of inefficiency, low life expectancy, loss of social support and family-related plans, marriage, And the high cost of living that is common in hemodialysis patients (26). Since depression is a chronic disease, it seems that the one-month intervention in this study was inadequate. It requires an extended duration to properly examine the effectiveness of cool dialysate on depression.
In this study, dialysis patients were sampled, and the intervention was performed in the COVID-19 peak. The COVID-19 disease has been the greatest public health emergency for international communities since 2020 (27). Along with physical health concerns, COVID-19 can cause mental distress (28). Symptoms of depression, anxiety, and stress caused by this epidemic have been evident globally, especially in patients dealing with chronic conditions. There were reports of suicide in some countries such as South Korea and India due to the anxiety of COVID-19 (29). Meanwhile, the media have increased the severity of fear and anxiety by frequently announcing death rates and hospitalizations (27). Psychological reactions can affect individuals’ behaviors and increase their psychological problems in times of emergencies (29). Therefore, cool dialysate could be ineffective due to the high levels of anxiety and depression in hemodialysis patients due to the COVID-19 pandemic.
Due to the COVID-19 pandemic, the emergency data collection made communication difficult for both the patient and the researcher. Besides, the pandemic itself was a limiting factor of the study. Finally, a one-month intervention seemed to be inadequate for the assessment of the cool dialysate effect.
5.1. Conclusions
The results of the study indicated that the application of cool dialysate was ineffective in reducing the severity of depression in hemodialysis patients, but despite the ineffectiveness of this method, the severity of depression intensified by stopping the intervention. It can be inferred that a difference between the two groups could be identified by a prolonged intervention. Therefore, researchers are advised to perform the intervention over a more extended period of six to 12 months to evaluate its effectiveness.