1. Background
Systemic lupus erythematosus (SLE) is a multisystemic disease with diverse pathogenesis and clinical presentations with considerable comorbidities and significant drug side effects (1-3). Understanding the root cause of SLE is still incomplete. Evidence suggests that abnormalities in the genetic, hormonal, immune system and environmental factors are related to the pathogenesis of SLE (4-6). Childhood-onset SLE (cSLE) is an SLE onset before the age of 18, which is associated with more serious multi-organ dysfunction compared to that of adults (6). The common hematological manifestations of cSLE are hemolytic anemia, thrombocytopenia, leukopenia, and lymphopenia (7).
SLE is a chronic inflammatory disease that affects the kidneys in about 50% of adult patients and about 80% of children (8-10). Lupus nephritis is one of the most common manifestations of SLE patients. It often leads to end-stage kidney disease (ESRD) for both adults and children (8). In patients with cSLE, not only clinical events are often more severe, but also multi-organ dysfunction is more common than SLE in adults (11-14). There is no publication concerning the demographic, clinical, and pathological features of pediatric LN and their correlations in Viet Nam. Therefore, the present study intended to evaluate some clinical and laboratory manifestations of Vietnamese pediatric patients with biopsy-proven LN class according to the International Society of Nephrology and the Renal Pathology Society (ISN/RPS) 2003 classification (revised in 2018), renal pathological activity, and chronicity index in children with LN.
2. Objectives
The current study intended to determine whether serum albumin level and urine protein/creatinine rate (uPCR) are appropriate predictors of severe lupus nephritis in childhood-onset SLE.
3. Methods
3.1. Study Design
The current cross-sectional, single-center-based study was performed at the Nephrology and Dialysis Department in National Children Hospital, Ha Noi, Viet Nam, from 6/2019 to 6/2020. A total of 85 children younger than 18 with presented clinical features of LN were recruited. SLE was diagnosed using the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) criteria for SLE classification (15). We defined LN as the 24-hour urinary protein ≥ 500 mg (or uPCR ≥ 0.5 mg/mmol) or the appearance of red blood cell casts in urine (> 5 RBC/HPF by manual analysis of the urine sediment) (16). Participants with other comorbidities, history of kidney transplantation, those receiving hemodialysis, primary neoplastic conditions, and inconclusive results were excluded.
3.2. Clinical and Laboratory Dataset
For each participant, the following laboratory data were collected: full blood count, immuno-biological tests (blood glucose, serum albumin, serum protein, serum C-reactive protein, serum double-stranded DNA (dsDNA), and serum C3 and C4 levels. Clinical parameters (age, sex, skin lesions, rheumatism, neurological lesion, and heart lesion) were also documented for each subject. The systemic lupus erythematosus disease activity index (SLEDAI) was calculated for all patients to determine SLE activity levels (15).
Parameters of kidney lesions were collected for each of the patients as: edema, hypertension, urine analysis, urine protein/creatinine rate, estimated glomerular filtration rate (eGFR). Hypertension is defined as blood pressure higher than the 95th percentile value of healthy people of the same age and sex (17). The eGFR was calculated based on the Schwartz formula (18). An eGFR < 60 ml/min per 1.73 m2 was considered as ESRD. We defined nephrotic syndrome as proteinuria > 50 mg/kg in 24h urine and serum albumin < 30 g/L. The LN patients with proteinuria < 50 mg/kg in 24h urine were classified as nephritis syndrome.
All 85 patients underwent renal biopsy. The indications of kidney biopsy were proteinuria > 500 mg/24h, active urine sediment (≥ 5 RBC or WBC/HPF, mostly dysmorphic without evidence of infection), or rising serum creatinine (19, 20). Renal biopsies were done by a Tru-cut semi-automated renal biopsy gun. Hematoxylin and Eosin (HE), periodic acid Schiff (PAS), silver methamine, Masson’s Trichrome (MT) stains were used for light microscopy. Specimens for immunofluorescence microscopy were received in Michelle’s medium and stained using fluorescein isothiocyanate (FITC) conjugated polyclonal rabbit antisera against human IgG, IgM, IgA, C3c, C1q, kappa, and lambda. Control slides were also examined simultaneously. The trained pathologists at our hospital examined all renal biopsy specimens. Histopathology classification of lupus nephritis was performed using six classes (i.e., I to VI) as the criteria of the International Society of Nephrology and the renal pathology society (ISN/RPS) in 2003 revised in 2018 (16).
3.3. Statistical Analyses
We represented continuous data by the mean and standard deviation (with normal distribution data) or median and interquartile range (with non-normal distribution). In addition, categorical data using frequency and percentage. Receiver operating characteristic (ROC) curves were analyzed to predict severe active LN. Data analysis was administered using SPSS version 20.0 (Chicago, IL, USA). Statistical significance was considered when P-value < 0.05.
4. Results
As shown in Table 1, SLE symptoms presented in patients with lupus nephritis were not considerable. The highest rate was skin lesion (57.6%), followed by rheumatism (29.4%) and the neurological lesion (10.6%). The proportion of patients with a decrease in WBC was 17.6%, while 75.3% of patients had anemia. The rate of patients with decreased serum albumin was 55.3%, increased CRP was 25%, increased Anti-dsDNA was 93.2%, decreased C3 was 90.6%, decreased C4 was 97.6%, and positive antinuclear antibodies accounted for 81.8%. Most patients had SLEDAI scores in moderate to very high activity.
| Clinical Characteristics and Laboratory Parameters | Values |
|---|---|
| Ages, y | 11.28 ± 2.21 |
| Gender | |
| Female | 73 (85.9) |
| Male | 12 (14.1) |
| BMI | |
| Mean | 17.71 ± 3.86 |
| Underweight | 15 (17.6) |
| Normal | 56 (65.9) |
| Overweight and Obesity | 14 (16.5) |
| Clinical features | |
| Skin lesion | 49 (57.6) |
| Mouth erosion | 15 (17.6) |
| Rheumatism | 25 (29.4) |
| Neurological lesion | 9 (10.6) |
| Heart lesion | 2 (2.4) |
| Full blood count | |
| RBC, T/L | 3.71 ± 0.99 |
| WBC, g/L: Median | 6.62 (4.59 - 10.01) |
| < 4.0 | 15 (17.6) |
| Hemoglobin, g/L: mean | 99.94 ± 22.82 |
| Anemia | 64 (75.3) |
| Platelet, g/L | 219 (149 - 320) |
| Neutrophil/lymphocyte rate | 2.15 (1.45 - 3.25) |
| Immuno-biological data | |
| Protein, g/L: Median | 61.7 (51.95 - 71.05) |
| < 56 | 28 (32.9) |
| Albumin, g/L: Mean | 28.55 ± 6.9 |
| < 30 | 47 (55.3) |
| CRP, mg/L: Median | 1.43 (0.32 - 5.54) |
| > 5 | 15 (25) |
| Anti-DsDNA, IU/L: Median | 378 (110.2 - 1316) |
| < 30 | 5 (6.8) |
| ≥ 30 | 68 (93.2) |
| C3, g/L: Median | 0.33 (0.25 - 0.54) |
| < 0.75 | 77 (90.6) |
| ≥ 0.75 | 8 (9.4) |
| C4, g/L: Median | 0.035 (0.01 - 0.079) |
| < 0.2 | 83 (97.6) |
| ≥ 0.2 | 2 (2.4) |
| Antinuclear antibodies (+) | 63 (81.8) |
| SLEDAI score | |
| Mean | 14.69 ± 4.45 |
| Mild activity | 0 (0) |
| Moderate activity | 18 (21.2) |
| High activity | 52 (61.2) |
| Very high activity | 15 (17.6) |
Abbreviations: Anti-dsDNA, anti-double-stranded deoxyribonucleic acid; BMI, body mass index; CRP, C reactive protein; C3, complement component 3; C4, Complement component 4; RBC, red blood cell; SLEDAI, the systemic lupus erythematosus disease activity index; WBC, white blood cell.
aValues are expressed as mean ± SD, or median (%), No. (%).
As shown in Table 2, those with clear LN with edema, hypertension, and oliguria, accounting for 34.1% to 63.5% of all participants. Up to 76.5% of patients had an increase in UPCR ≥ 200 mg/mmol. With the clinical syndromes of lupus nephritis, nephrotic syndrome accounted for 51.8% of patients, 7.1% of participants had kidney failure.
| Some Characteristics of Lupus Nephritis | Values |
|---|---|
| Edema | 54 (63.5) |
| Hypertension | 29 (34.1) |
| Oliguria | 62 (61.2) |
| Urine analysis | |
| Hematuria (+) | 69 (81.2) |
| Proteinuria (+) | 85 (100.0) |
| Leukocyte (+) | 38 (44.7) |
| uPCR, mg/mmol | |
| Median | 446.6 (241.89 - 799.02) |
| < 200 | 20 (23.5) |
| ≥ 200 | 65 (76.5) |
| Clinical syndromes | |
| Nephritic syndrome | 35 (41.1) |
| Nephrotic syndrome | 44 (51.8) |
| Renal failure | 6 (7.1) |
| eGFR, mL/min/1.73 m2 | |
| Mean | 106.49 ± 34.72 |
| < 60 | 6 (7.1) |
Abbreviations: eGFR, estimated glomerular filtration rate; UPCR, urine protein/creatinine rate.
aValues are expressed as mean ± SD, or median (%), No. (%).
According to pathological morphology, most patients had an LN class of III and IV (37.6% for each class), and only 7.1% had an LN class of V and 17.6% class II. No patient was from a class I and VI (Table 3).
| Classification | No. (%) |
|---|---|
| Class I: Minimal mesangial lupus nephritis | 0 (0) |
| Class II: Mesangial proliferative lupus nephritis | 15 (17.6) |
| Class III: Focal lupus nephritis | 32 (37.6) |
| Class IV: Diffuse Lupus Nephritis | 32 (37.6) |
| Class V: Membranous lupus nephritis | 6 (7.1) |
| Class VI: Advanced sclerotic lupus nephritis | 0 (0) |
As shown in Table 4, the serum albumin decreased gradually, while the rate of nephrotic syndrome, uPCR, and SLEDAI score increased gradually from LN class II to V. The WBC, hemoglobin level, Anti-dsDNA, as well as eGFR, changed irregularly, and there was a statistically significant difference between the study groups (P < 0.05).
| Characteristics | Class II (N = 15) | Class III (N = 32) | Class IV (N = 32) | Class V (N = 6) | P |
|---|---|---|---|---|---|
| Ages, y | 12.27 ± 1.33 | 10.75 ± 2.15 | 11.44 ± 2.24 | 10.83 ± 3.54 | 0.157 |
| Gender | |||||
| Male | 14 (93.3) | 26 (81.3) | 28 (87.5) | 5 (83.3) | 0.716 |
| Female | 1 (6.7) | 6 (18.8) | 4 (12.5) | 1 (16.7) | |
| BMI | 17.18 ± 4.58 | 17.24 ± 3.34 | 17.91 ± 3.35 | 20.5 ± 6.45 | 0.269 |
| FBC | |||||
| WBC, g/L | 6.73 (4.73 - 12.13) | 5.7 (3.56 - 9.33) | 5.96 (4.62 - 8.75) | 14.02 (8.39 - 17.35) | 0.043 |
| Hemoglobin, g/L | 109.13 ± 27.73 | 100.25 ± 18.99 | 92.12 ± 19.3 | 117 ± 32.49 | 0.02 |
| Platelet, g/L | 235 (149 - 380) | 189.5 (132.75 - 307) | 223 (155 - 335.75) | 293 (195.75 - 301.3) | 0.612 |
| Immuno- | |||||
| Biological data | |||||
| Albumin, g/L | 31.31 ± 7.6 | 30.71 ± 6.21 | 26.42 ± 6.19 | 21.53 ± 4.57 | 0.001 |
| CRP, mg/L | 2.41 (0.33 - 3.17) | 0.94 (0.32 - 7.96) | 1.24 (0.34 - 4.09) | 6.39 (0.8 - 16.25) | 0.839 |
| Anti-DsDNA, IU/L | 163 (42.9 - 381.42) | 1200 (225.95 - 2170) | 491.15 (212.62 - 1507.75) | 96.85 (67.85 - 449.07) | 0.003 |
| C3, g/L | 0.45 (0.29 - 0.72) | 0.33 (0.21 - 0.52) | 0.3 (0.24 - 0.42) | 0.54 (0.44 - 0.7) | 0.105 |
| C4, g/L | 0.02 (0.01 - 0.08) | 0.03 (0.02 - 0.08) | 0.04 (0.01 - 0.06) | 0.05 (0.01 - 0.08) | 0.751 |
| Edema | 7 (46.7) | 15 (46.9) | 18 (56.3) | 4 (66.7) | 0.74 |
| Hypertension | 3 (20) | 7 (21.9) | 16 (50) | 3 (50) | 0.052 |
| Nephrotic syndrome | 4 (26.7) | 12 (37.5) | 22 (68.8) | 6 (100) | 0.001 |
| uPCR, mg/mmol | 296.22 (90 - 446.6) | 339.39 (121.6 - 537.6) | 641.28 (389.8 - 993.3) | 1432.45 (1028.7-3290.9) | < 0.001 |
| eGFR, mL/min/1.73 m2 | 121.83 ± 28.6 | 114.25 ± 27.5 | 87.76 ± 33.52 | 126.58 ± 49.85 | 0.001 |
| SLEDAI score | 11.93 ± 3.88 | 14.97 ± 4.82 | 15.28 ± 3.69 | 17 ± 5.47 | 0.042 |
Abbreviations: Anti-dsDNA, anti-double-stranded deoxyribonucleic acid; BMI, body mass index; CRP, C reactive protein; C3, complement component 3; C4, complement component 4; eGFR, estimated glomerular filtration rate; FBC, full blood count; uPCR, urine protein/creatinine rate; SLEDAI, the systemic lupus erythematosus disease activity index; WBC, white blood cell.
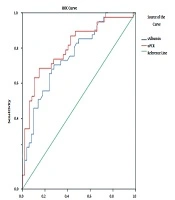
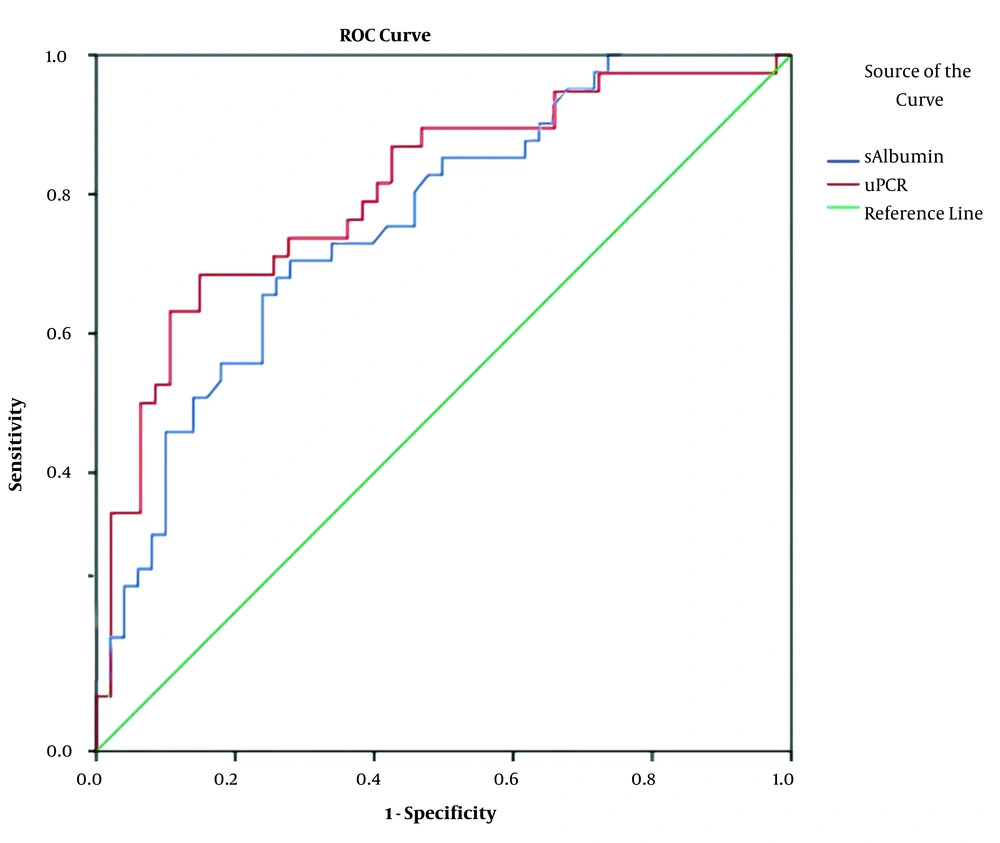
Based on the Receiver operating characteristics (ROC) curve model (Figure 1), serum albumin, uPCR were appropriate predictors for severe, active LN (classes IV and V) (Serum albumin: AUC = 0.725, P < 0.001, Cut-off value: 28.65 g/L, Se = 68.4%, Sp = 70.2%. uPCR: AUC = 0.725, P < 0.001, Cut-off value: 558.56 mg/mmol, Se = 68.4%, Sp = 85.1%).
5. Discussion
Lupus nephritis (LN) is a common manifestation among SLE patients, with a prevalence of 60% in adults and 80% in children. Up to 30% of patients progress to end-stage renal disease (21). In our study, LN’s clinical and laboratory characteristics are consistent with other studies conducted in various countries (22, 23). In addition, in the present study, the female-to-male ratio was 6.08/1, with diverse clinical manifestations of SLE. Patient with rheumatism and skin lesion accounted for the highest proportion (57.6% and 29.4%, respectively), while the neurological lesion rate accounted for 10.6%. The proportion of anemia is 75.3%, and the average SLEDAI score is up to 14.69 point. Relevant immunological markers were encountered in the majority of cases, with 81.8% positive for antinuclear antibodies, 93.2% increase in Anti-Ds-DNA ≥ 30 UI/L, 90.6% decrease in C3 < 0.75 g/L, and 97.6% decrease in C4 < 0.2 g/L. Local deposition of immune complexes, a consequence of classical activation of the complement, leads to decreased complement system protein levels (14, 24, 25). Many studies reported that decreased C3 and C4 concentrations in plasma are related to the disease’s active state (14, 26). According to the findings of the present study, patients with LN have severe clinical manifestations that demonstrate the mechanism of multiple organ damage of active SLE. Research on the pathogenesis of SLE showed an interaction between genetic and environmental factors, thereby impairing immune tolerance and initiating chronic autoimmune disease (27, 28). Many studies have shown that SLE clinical manifestations in children are often more severe and more susceptible to multiple organ damage than SLE in adults (11, 12, 14, 24).
Carrying out a study on 85 children with LN, we found that kidney lesions’ clinical and subclinical symptoms were evident with 63.5% edema, 61.2% oliguria, and 34.1% hypertension. Urine analysis showed 81.2% positive hematuria and 100% positive proteinuria. Several studies indicated that hematuria and proteinuria are the most common kidney damage abnormalities (67 to 100% of SLE children) (11, 29). Various studies reported that hypertension accounts for 30 to 50% (11, 22). In our study, the ratio of patients with nephrotic syndrome was 51.8%, similar to Batinic et al. (30), but renal failure patients were 7.1%, which is lower than the rate reported by Sevinc et al. (31). In patients with LN, the glomerulus is the most severely damaged structure (14). Changes in membrane permeability are often related to proteinuria, local inflammation, glomerular hematuria, and decreased glomerular filtration (14). Regarding histopathological results, in the present study, the proportion of patients with class III and IV LN was 75.2%, which is in line with the results of some other studies (32, 33). In LN patients, the production of systemic autoantibodies and complement disturbances are common. The immune complex deposited in glomeruli results in podocyte, mesangial cell, endothelial cell injury. When comparing the clinical and subclinical characteristics of LN classes, we found a gradual decline in the serum albumin concentration, the proportion of patients with nephrotic syndrome together with the median uPCR and SLEDAI score gradually increased from LN class II to V with P < 0.05 (Table 4). Our results are consistent with some of the previously conducted studies and the pathogenetic mechanism of the kidney damage of LN (9, 12, 16, 22, 23).
A kidney biopsy is necessary to monitor the progression of LN. However, first, the patients must be hospitalized, and if the biopsy is available, techniques to diagnose pathological morphology should be provided immediately. Besides, those with severe diseases can not always undergo renal biopsies. An important question is that whether it is possible to use clinical manifestations or subclinical tests to preliminarily diagnose the class of LN to provide appropriate treatment immediately after hospitalization? We hypothesized that a decrease in serum albumin and an increase in uPCR could predict LN classes of IV and V. The results showed that both serum albumin and uPCR are valid for predicting LN classes pf IV and V, in which uPCR has a better value with AUC = 0.725, P < 0.001, Cut-off value: 558.56 mg/mmol, Se = 68.4%, Sp = 85.1% (Figure 1). Although predictable, clinical practice shows that it is complicated to treat hypertension, massive proteinuria, and impaired function. LN classes of IV and V, and even class III must be treated even when the patient cannot undergo a renal biopsy (8, 34, 35).
5.1. Conclusions
Serum albumin and uPCR were good predictors for severe, active LN.