1. Background
Chronic kidney disease (CKD) is the leading cause of disability and mortality. It is an irreversible pathologic process resulting in an unstopped decrease in the number and function of nephrons. In most cases, it may lead to end-stage renal disease (ESRD) and permanent dependence on renal replacement therapies such as peritoneal dialysis (1). Accordingly, the prevalence of CKD is increasing worldwide (2). According to the reports issued in 2009, approximately 20 million persons in the USA suffer from CKD (3, 4).
In recent years, the prevalence and the emergence of ESRD have increased in Iran (5-7). Cardiovascular diseases (CVD) are more prevalent in these patients as their prevalence is 10 - 20 times much more frequent than the normal population (8, 9). Before affection by ESRD, patients with CKD may pass away or suffer from CVD, as the leading cause of death in CKD patients (4). The mortality and morbidity of CVD are extremely more common in ESRD patients compared to the normal population (10).
The inflammation in the vessel wall plays a critical role in atherosclerosis progression, plaque injury, and rupture of atherosclerotic plaques (11). Today, increased permanent inflammation and oxidative stress in patients undergoing regular dialysis therapy are well-documented (12). Chronic inflammatory conditions have been widely noticed in the ESRD patients undergoing dialysis. Oxidative stress and endothelial dysfunction may promote inflammatory responses, which plays a vital role in the atherosclerosis process development and thus links CKD to CVD (4).
The C-reactive protein (CRP) is one of the most sensitive acute-phase proteins produced by the hepatocytes and under the influence of cytokines such as IL-6 and TNF-α. Its serum level is low in the absence of active diseases raising after the inflammatory response (13, 14). Accordingly, CRP is easily measured, and its normal range is 3 - 5 mg/L (15). Different values in the normal range are measured by high sensitivity CRP (hsCRP), and its normal values are considered to be 0.2 - 5 mg/L (16).
Using antioxidants is considered as an effective strategy to reduce oxidative stress in CKD patients (17). The role of different antioxidants in reducing oxidative stress in these patients have been examined, among which N-Acetyl cysteine (NAC) is known as a valuable and safe antioxidant and anti-inflammatory drug. This drug is commonly used as the antidote to acetaminophen poisoning, a mucolytic drug to treat chronic bronchitis. It is also used in preventing contrast-induced nephropathy and is well-tolerated with no severe side-effects (9, 17, 18). Furthermore, NAC plays a protective role in the heart against oxygen-free radicals (19). During oxidative stress, glutathione concentrations decrease, and NAC acts as an antioxidant by increasing glutathione (18). Moreover, the anti-inflammatory effects of NAC are reported in previous studies. The drug inhibits lipopolysaccharide-induced lipid peroxidation, proinflammatory cytokines, and nitric oxide release. Further, it may inhibit the proinflammatory transcription factor NF-kappa B; hence, the expression of several proinflammatory genes is down-regulated (19). However, the antioxidant and anti-inflammatory effects of NAC on continuous ambulatory peritoneal dialysis (CAPD) patients are still unclear (17, 20, 21).
2. Objectives
This study aimed to assess the effects of NAC on the hsCRP level in patients on CAPA.
3. Methods
3.1. Study Design and Setting
The present study was a quasi-experimental self-controlled study conducted from March 2012 to July 2014 and included the CKD patients under CAPD, who referred to the peritoneal dialysis clinic at the Shahid Sadoughi Hospital, one of the general and teaching hospital affiliated to the Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3.2. Study Population and Sample Size
Inclusion criteria were adults aged above 18 years with a confirmed diagnosis of CKD who were on CAPD for at least six months. Moreover, patients would be excluded from the study if they had inflammatory diseases such as rheumatoid arthritis and SLE, active infection, hepatitis type B or C, tunnel and exit site infections, peritonitis, and CRP > 50 mg/L (22). The sample size was estimated to be 46 persons (α = 0.05 and β = 80%) (23); however, it was considered to be 50 patients in the present study.
3.3. Study Protocol and Intervention
According to the mean level of the last 3 CRP levels, which were measured each month for the patients in this center and recorded in their medical files, the participants were divided into two groups: CRP 5 - 50 mg/L (A) and CRP < 5 mg/L (group B). CRP can elevate by > 5 mg/L under some conditions and spontaneously decrease later. Accordingly, the CRP level was measured during three months (22). Table 1 shows the measurement methods of inflammatory markers.
| Inflammatory Marker | Normal Range, mg/L | Measurement Methods | Used Kit | Country |
|---|---|---|---|---|
| CRP | 3 - 5 | Torbidometery | Bionic | Iran |
| hsCRP | 0.2 - 5 | ELIZA | Evence 2300 | USA |
The data collection form addressed the patients’ age, gender, medical history, cause of ESRD, duration of dialysis, and CRP level. For both groups, hsCRP was measured before the intervention by considering blood sampling. Afterward, they were treated with oral NAC 600 mg × 2 daily for eight weeks, which was made by HEXAL Company in Germany. Following the treatment, hsCRP was measured for all the participants once more. During the intervention, all the patients followed the proper usage of drugs to avoid their adverse effects. Then the hsCRP was separately compared in each group before and after the intervention. It is worth mentioning that all blood samples (before and after the intervention) were frozen to assess hsCRP simultaneously.
3.4. Statistical Analysis
The data analyses were performed by SPSS software version 16.0 (SPSS Inc., Chicago, USA) for Windows, and the paired- and independent-sample t-test, one-way ANOVA, chi-square, Pearson, and Spearman’s Correlation Coefficient tests were used to compare the findings. The results are presented as mean ± standard deviation (SD) for continuous variables and are summarized in frequencies (percentage) for categorical ones. Two-sided P < 0.05 and confidence interval (CI) of 95% were set to be statistically significant.
4. Results
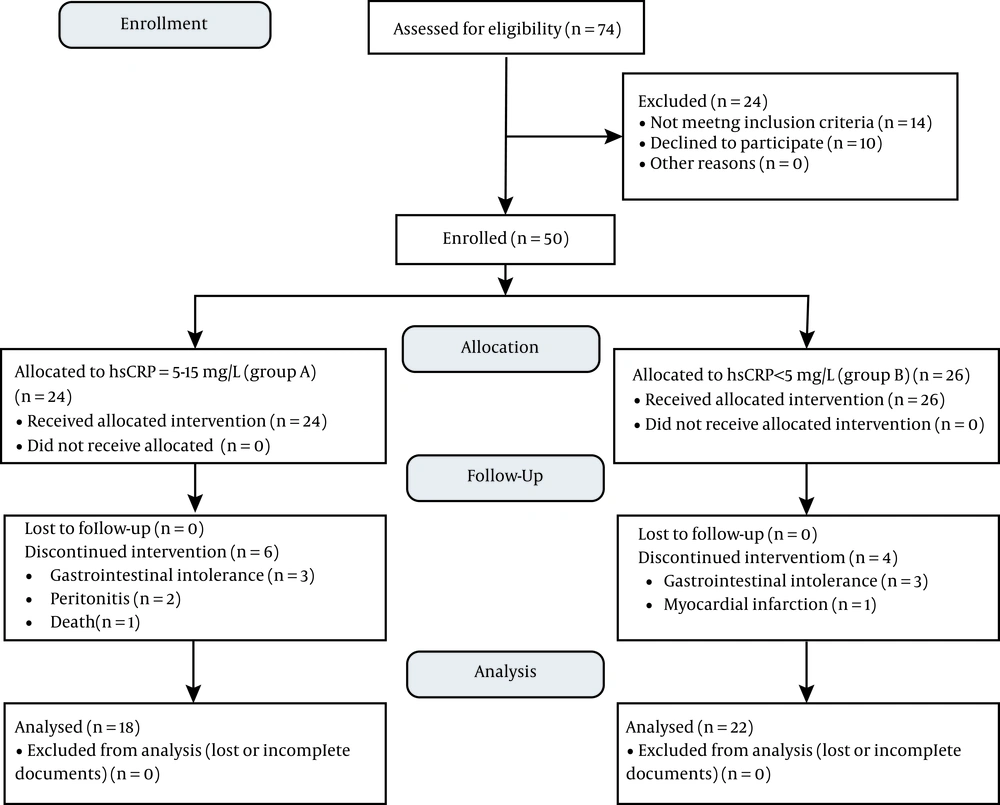
Of the 74 patients assessed for eligibility, 14 patients did not meet the inclusion criteria, and 10 individuals refused to participate in the study. Accordingly, the final sample size was 50 persons, among which 24 patients had the CRP level of 5 - 50 mg/L (group A), and 26 patients had CRP level < 5 mg/L (group B). Ten patients (20%) were excluded (six patients from group A and four patients from group B) mainly because of gastrointestinal intolerance (12%), peritonitis (4%), myocardial infarction (2%), and death (2%). Finally, the data obtained from 40 participants were analyzed [18 patients in the group A (mean ± SD; hsCRP = 7.75 ± 4.64), and 22 patients in the group B (mean ± SD; hsCRP = 2.42 ± 1.54)]. Figure 1 shows the CONSORT flow diagram.
The participants’ mean ± SD of age was 55.05 ± 12.94 years, and 22 patients (55%) were male. The mean ± SD of duration of dialysis was 30.95 ± 25.82 months, and diabetes mellitus (50%) was the leading cause of ESRD in these patients. All patients in the groups A and B were homogenous in terms of age, gender, underlying diseases, and duration of dialysis (Table 2).
| Variable | Total (N = 40) | Group A (CRP = 5 - 50 mg/L) (N = 18) | Group B (CRP < 5 mg/L) (N = 22) | P-Value | 95% Confidence Interval |
|---|---|---|---|---|---|
| Age, y | 55.05 ± 12.94 | 53.56 ± 12.43 | 56.27 ± 13.51 | 0.516 | -11.11, 5.67 |
| Men | 57.45 ± 13.80 | 55.33 ± 11.88 | 60.13 ± 13.85 | ||
| Women | 52.11 ± 11.50 | 55.17 ± 12.90 | 46.00 ± 4.10 | ||
| P-value | 0.198 | 0.454 | 0.025* | ||
| Gender | 0.24 | - | |||
| Men | 22 (55) | 6 (33.3) | 16 (72.7) | ||
| Women | 18 (45) | 12 (66.7) | 6 (27.3) | ||
| P-value | 0.374 | 0.535 | 0.293 | ||
| Underlying disease | 0.113 | - | |||
| Diabetes mellitus | 20 (50) | 10 (55.6) | 10 (45.5) | ||
| Hypertension | 6 (15) | 4 (22.2) | 2 (9.1) | ||
| Renal stone | 4 (10) | 2 (11.1) | 2 (9.1) | ||
| Glomerulonephritis | 2 (5) | 0 (0) | 2 (9.1) | ||
| Congenital disease of urinary system | 2 (5) | 2 (11.1) | 0 (0) | ||
| Cystic disease | 2 (5) | 0 (0) | 2 (9.1) | ||
| Unknown | 4 (10) | 0 (0) | 4 (18.2) | ||
| Duration of dialysis, mo | 30.95 ± 25.82 | 28.78 ± 22.01 | 32.73 ± 28.92 | 0.636 | -20.73, |
| Men | 35.73 ± 28.07 | 32.33 ± 5.09 | 37.00 ± 32.99 | 12.83 | |
| Women | 25.11 ± 22.14 | 27.0 ± 27.02 | 21.33 ± 5.96 | ||
| P-value | 0.20 | 0.643 | 0.086 | ||
| First hsCRP, mg/L | 4.31 ± 4.49 | 7.75 ± 4.64 | 1.49 ± 1.26 | < 0.001* | 3.90, 8.62 |
| Men | 3.56 ± 4.49 | 10.02 ± 3.59 | 1.14 ± 0.98 | ||
| Women | 5.22 ± 4.46 | 6.61 ± 4.82 | 2.42 ± 1.54 | ||
| P-value | 0.253 | 0.147 | 0.031* | ||
| Second hsCRP, mg/L | 2.99 ± 3.73 | 5.52 ± 4.35 | 0.92 ± 0.70 | < 0.001* | 2.42, 6.78 |
| Men | 3.0 ± 4.05 | 8.87 ± 3.35 | 0.79 ± 0.50 | ||
| Women | 2.98 ± 3.40 | 3.84 ± 3.87 | 1.26 ± 1.06 | ||
| P-value | 0.991 | 0.015* | 0.167 | ||
| hsCRP difference (second-first) | 1.32 ± 0.99 | 2.23 ± 1.23 | 0.57 ± 0.75 | 0.013* | 0.388, 2.93 |
| Men | 0.57 ± 0.71 | 1.15 ± 0.55 | 0.35 ± 0.64 | ||
| Women | 2.23 ± 2.50 | 2.77 ± 2.91 | 1.16 ± 0.73 | ||
| P-value | 0.002* | 0.086 | 0.02* |
aValeues are expressed as mean ± SD or No. (%).
b*, Statistically significant.
As expected, the first and the second hsCRP were higher in the group A (P < 0.001). The result of the paired-sample t-test in each group revealed that NAC could reduce the hsCRP level in both groups (P = 0.001 in group A vs. P = 0.002 in group B); however, the decrease was more prominent in the group A (P = 0.013). Further analysis revealed that the hsCRP decrease was more significant in women (P = 0.002) in general and women in the group B (P = 0.02) in particular. The hsCRP variation had a significant relationship with the underlying disease (P = 0.009). No relationship was observed in the group A (P = 0.28); however, it was statistically significant in terms of hypertension in the group B (P = 0.001).
As shown in Table 3, there was no linear correlation between hsCRP variation and age in general (r = -0.173, P = 0.285) in both group A (r = -0.198, P = 0.431), and group B (r = -0.209, P = 0.350). Moreover, there was no correlation between the hsCRP variation and the duration of dialysis. In this regard, such a correlation was not observed in the first and second hsCRP.
| Variables | First hsCRP | Second hsCRP | hsCRP Difference (Second-First) | |||
|---|---|---|---|---|---|---|
| P-Value | r | P-Value | r | P-Value | r | |
| Age | ||||||
| Total | 0.634 | -0.078 | 0.949 | -0.01 | 0.285 | -0.173 |
| A | 0.83 | 0.054 | 0.499 | 0.171 | 0.431 | -0.198 |
| B | 0.22 | -0.272 | 0.231 | -0.266 | 0.350 | -0.209 |
| Gender | ||||||
| Total | 0.253 | - | 0.991 | - | 0.002* | - |
| A | 0.147 | - | 0.015* | - | 0.086 | - |
| B | 0.031* | - | 0.167 | - | 0.020* | - |
| Duration of dialysis | ||||||
| Total | 0.625 | 0.080 | 0.391 | -0.139 | 0.621 | 0.081 |
| A | 0.185 | -0.327 | 0.469 | -0.182 | 0.241 | -0.291 |
| B | 0.786 | 0.062 | 0.639 | -0.106 | 0.366 | 0.203 |
a*, Statistically significant.
5. Discussion
The present findings indicated that NAC could significantly reduce the hsCRP level in CAPD patients on both low and high CRP levels. This reduction was more prominent in the group A (CRP of 5 - 50 mg/L). This decrease was observed during the 8-week use of oral NAC (600 mg, twice a day), suggesting that NAC reduced the hsCRP level in patients with higher hsCRP. What distinguishes our study from other studies (9, 17, 24, 25) is the classification of the patients into two groups (namely those with CRP < 5 mg/L and those with CRP = 5 - 50 mg/L), resulting in more accurate results to detect the effectiveness of NAC in a low CRP level in decreasing systemic inflammation.
Different antioxidants have been assessed in previous studies to reduce oxidative stress such as vitamin E, NAC, and L-carnitine in the CKD patients, according to which the use of NAC reduces inflammatory factors (such as IL1, TNFα, etc.), decrease the risk of CVD and its protection against oxygen-free radicals (9, 10, 21). However, side effects such as gastric intolerance, including nausea and vomiting, rash, and itching for NAC, have also been reported (17). Moreover, 10 patients (20%) were excluded from the final analysis in this study due to adverse events caused by drugs.
Nascimento et al. (9), in a case-control study on 30 peritoneal dialysis patients receiving 600 mg of NAC twice a day for eight weeks and those receiving placebo revealed no significant effect of NAC on inflammatory markers, except for the blood level of IL6. However, the present study examined a larger number of patients, and the findings were in contrast.
The double-blind clinical trials with placebo by Swarnalatha et al. (24) on 14 hemodialysis patients receiving 600 mg of NAC twice a day for 10 days before iron therapy indicated that NAC reduced hsCRP level; however, the decrease was not significant. Following the iron therapy, the hsCRP level slightly increased. In their study, NAC could only reduce the level of 3,4-Methylenedioxyamphetamine (MDA) as an inflammatory factor. In contrast, the findings of our study showed the significant effects of this drug on reducing the hsCRP level. Unlike the present study’s findings, no adverse drug reaction was observed in their study.
In a study by Purwanto and Prasetyo (17), 32 patients under CAPD received 600 mg of NAC twice a day for eight weeks (similar to our study). The patients were divided into two 16-member groups. Then the inflammatory factors (namely IL1, IL6, hsCRP, SICAM, leukocyte, and TNFα) were measured. The results revealed that the short-term use of this drug in the treatment group reduced the hsCRP levels (P < 0.001) and other inflammation markers. The same finding was observed in the present study. Moreover, the present findings also considered age, gender, and dialysis duration. The authors also published no data in terms of adverse drug reactions in their patients.
Saddadi et al. (25) investigated 24 hemodialysis patients with 600 mg of NAC twice a day for three months and asked the patients to avoid taking medications with antioxidant effects during the study. Itching was reported as the only side-effect of the drug. A significant inverse relationship was observed between hsCRP variations and age; however, such a relationship was not significant in the present study. One of the similarities between this study and our study is the relationship between the hsCRP variation and the duration of dialysis, which was not significant in both studies. Similar to our findings, their findings indicated a further decrease in the hsCRP after receiving NAC in women (P = 0.04). They also suggested that BMI and dietary factors may cause changes in the CRP levels.
Marques-Vidal et al.’s (26) study on 6,000 Swiss patients (2884 men and 3201 women) in the range age of 35 - 75 years indicated that the CRP level was positively correlated with age, body mass index, and smoking and inversely correlated with physical activity. Moreover, they showed that the CRP level was inversely correlated with gender (in favor of males); however, in general, the CRP level was higher in males. We found that the hsCRP variations were more remarkable in women and that the variation was also more noticeable in the group B. Although the reason for such difference is still unknown, some studies have referred to the critical role of sex hormones in the CRP levels and other inflammatory mediators. Norouzi et al. (27) stated that the three-month use of oral contraceptives could significantly increase the CRP levels and homocysteine, and the progestin-enhanced IL-6 stimulation mechanism of producing CRP may be caused by its effects on IL-6 receptors, especially glycoprotein 130 or the direct augmentation of the intracellular CRP production.
Remarkably, no similar study has documented the association between the hsCRP level and its variations with the underlying diseases. This issue was thoroughly examined in the present study. The findings revealed that if the patients were assessed in a general state, the hsCRP level and its variations would be associated with the underlying diseases. However, if the patients were assessed in each group, the relationship between CRP < 5 mg/L and hypertension was only significant. This is while diabetes mellitus was the most common underlying disease among these patients, and its relationship with this underlying disease was expected. However, it should be noted that this finding was not sufficiently reliable due to the limited number of patients in each group.
The exclusion of some patients, mainly due to the adverse drug events, and a decrease in the sample size, was the main limitation of the study, especially in determining the relationship between the hsCRP variation and the underlying disease. The high price of the hsCRP assay kit and uncommon use of this kit in laboratories are assumed as further limitations in including a larger sample. However, if this test was used routinely and was less expensive, there would be no need to freeze blood samples before and after the treatment of the blood samples to assess them simultaneously. This problem also aroused time limitation for the study because the frozen blood samples had to be tested during 4 - 5 months, and consequently, this made replacing the excluded patients impossible. Furthermore, given this limitation, the third investigation of the hsCRP level to follow up the patients and draw the correlation coefficient graph was impossible.
Further studies on a larger-scale population are recommended to better clarify the relationship between the variations in the hsCRP level and underlying diseases and to show whether the underlying diseases affect the response to treatment. Moreover, it was found that NAC reduces the blood level of hsCRP; however, a long-term cohort study would be required to understand to what extent the drug reduces CVD. The findings revealed that 12% of the patients experienced drug-induced gastric intolerance; hence, the administration of lower dosages for a more extended period may solve this problem. To this end, further studies are recommended to delve into this issue.
5.1. Conclusions
Our findings revealed that the administration of NAC (600 mg, twice a day for eight weeks) could significantly reduce the hsCRP level as an inflammatory factor in patients on CAPD, especially in CRP of 5 - 15 mh/L. The decrease has no relationship with age and duration of dialysis; however, it was more prominent in women. Accordingly, if the patients reveal no symptoms of drug-induced gastric intolerance, oral NAC is recommended for all CAPD patients with hsCRP > 5 mg/L for systemic inflammation reduction and, consequently, CVD risk.