1. Introduction
By the end of 2020, more than 83 million confirmed cases of Coronavirus infection (COVID-19) and 1.8 million deaths occurred worldwide (1). Several studies reported the correlation between COVID-19 and inflammatory syndromes, such as Guillain-Barre, myocarditis, and encephalitis (2-4). Indeed, a significant proportion of mortalities and morbidities in COVID-19 is due to non-pulmonary immune-mediated complications. These syndromes may affect any organ and play an essential role in tissue damage and death (5). The cutaneous symptom is a relatively common presentation in COVID-19. It may occur at the onset of this disease or a few days later and usually have a favorable outcome. We reported a rare case of COVID-19 pneumonia who developed Henoch–Schoenlein purpura (HSP) on admission.
2. Case Presentation
The patient was a 21-old-year male referred to the emergency department with three days of fever, myalgia, shortness of breath, and dry cough, five days after close contact with a patient with COVID-19. He also complained of abdominal pain and hematuria for two days. Physical examination revealed a blood pressure of 92/55 mmHg, a pulse rate of 128 beats per minute, a respiratory rate of 28 per minute, an oral temperature of 38.6°C, and O2 saturation of 88%. He also had crackle in both lungs and a mild diffuse abdominal tenderness. The pulmonary computed tomography scan showed diffusely scattered ground-glass opacities. The lab tests showed a white cell count of 5300 per cubic millimeter (consisting of 83% of polymorphonuclears, 13% lymphocytes, and 4% monocytes), a hemoglobin level of 11.4 mg/dL, a platelet count of 314,000 per cubic millimeter, and a creatinine level of 1.1 mg/dL. The first-hour erythrocyte sedimentation rate level was 89, and the C-reactive protein level was 68 mg per liter. Urine analysis showed mild proteinuria and 20 - 25 red blood cells in a 40x magnification field with 30% dysmorphic. The nasopharyngeal swab for the SARS Coronavirus-2 polymerase chain reaction (PCR) was positive.
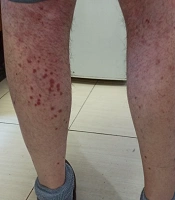
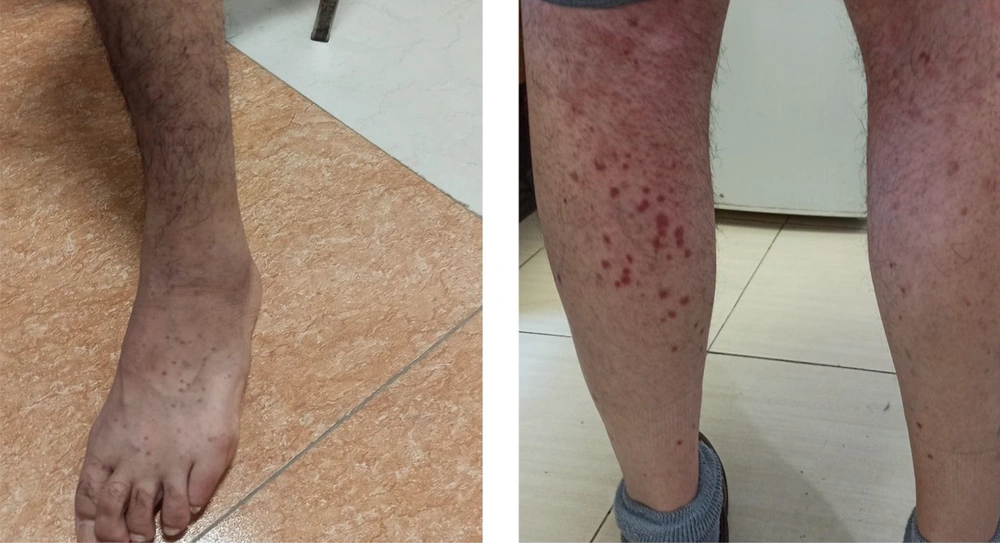
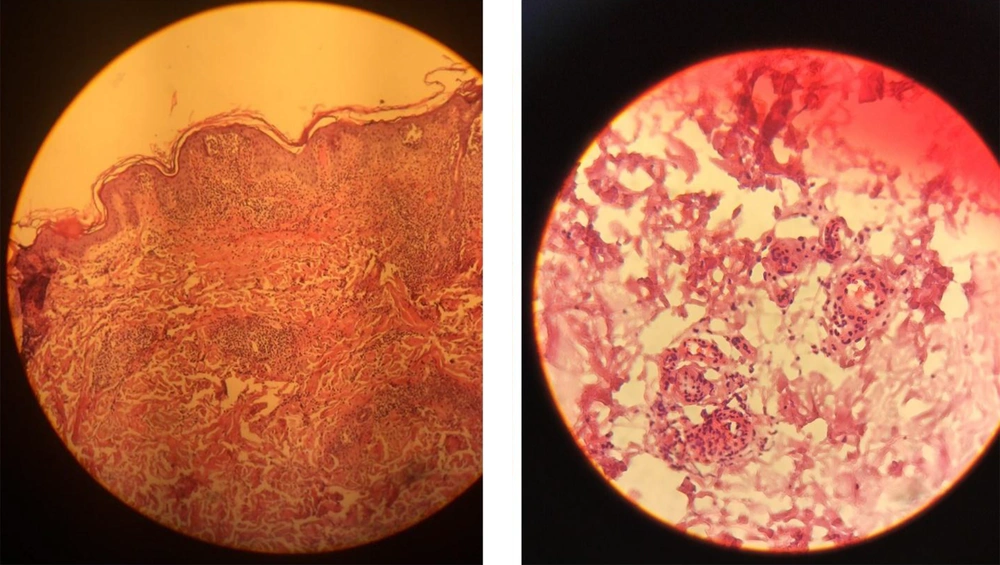
We started treatment with parenteral ceftriaxone and pantoprazole, oral azithromycin and lopinavir/ritonavir, and oxygen supplement. The pulmonary symptoms recovered gradually during the following days, but abdominal pain and malaise continued. We also asked for a nephrology consultation and complete abdominopelvic ultrasound for evaluating hematuria. The ultrasound study was unremarkable. Other laboratory tests showed a urine protein level of 565 mg in a day, negative results for the urine culture, anti-nuclear antibody, anti-double-stranded DNA, antineutrophil cytoplasmic antibodies, HBs antigen, HCV antibody, and HIV antibody. Also, D-Dimer, prothrombin and partial thromboplastin times, peripheral blood smear, and the serum complements were normal. In the second week of admission, a palpable purpuric rash appeared on his back and lower extremities and extended in two days (Figure 1). Upon consultation with a dermatologist and rheumatologist, we performed a skin punch biopsy and started intravenous dexamethasone 4 mg three times a day, which improved his condition in the following days. The histopathology study revealed superficial lymphocytic infiltrate and small-vessel vasculitis in the dermis (Figure 2). We diagnosed HSP based on clinical presentations and histopathological findings. Finally, we discharged him on day 18 of admission without any vital organs damage with oral prednisolone and ferrous sulfate supplement. The purpuric rashes disappeared after two weeks.
3. Discussion
Henoch–Schoenlein purpura is a small-vessel vasculitis, which mainly affects the skin and mucous membranes. It may develop after upper respiratory tract infections such as Streptococci, Adenoviruses, and coxsackievirus (6). In our case, HSP occurred after SARS Coronavirus-2 infection, which is a new manifestation of COVID-19. The cutaneous presentation of COVID-19 is often like the other viral exanthemas, such as maculopapular rash. Table 1 lists the different types of dermal histopathology in COVID-19. As we showed in the table, the most common cutaneous manifestations of COVID-19 are widespread maculopapular rashes, papulovesicular rashes, and acral cyanosis. Skin symptoms in COVID-19 are usually self-limited and leave with a favorable outcome, but severe dermal involvement such as vasculitis needs a systemic treatment. Physicians should consider COVID-19 in the differential diagnosis of a new-onset cutaneous symptom, even a rare dermal presentation such as vasculitis.
| Reference | Outcome | Histopathological Findings | Types of Skin Lesion | Age (Mean) | Female to Male | Number of Patients |
|---|---|---|---|---|---|---|
| (7) | Recovery | Intraepidermal vesicles with mild acantholysis and ballooned keratinocytes | Vesicular skin rash including palms and soles | 40.5 | 15/3 | 24 |
| (7) | Recovery | Intraepidermal vesicles with mild acantholysis and ballooned keratinocytes | Localized pattern (trunk) | 47.5 | 3/3 | 24 |
| (8) | Three death, twelve recovery | Hyperkeratosis, vacuolar degeneration of the basal layer with multinucleate hyperchromatic keratinocytes and dyskeratotic cells | Diffuse (73%) or scattered (27%) papulovesicular rash on trunk and limbs | 56.4 | 16/6 | 22 |
| (9) | Recovery | Spongiosis in epidermis. dilated vessels filled with neutrophils, extravasation of RBCs, lymphocytic perivascular and interstitial infiltrate | Erythema multiform-like exanthema, target lesions | 66.7 | F | 4 |
| (10) | Recovery | Superficial perivascular dermatitis, small thrombus, extravasated of RBCs | Widespread erythematous macules on arms, trunk, and lower limbs, exanthem on the trunk and arms | 68.3 | 2/1 | 3 |
| (11) | Recovery | Edema of the papillary dermis superficial and lymphocytic infiltrate | Chilblains or multiple asymptomatic erythematous and edematous, partially eroded, macules and plaques on dorsal aspects of the fingers | 16 | M | 1 |
| (9) | NA | Viral exanthem, thrombotic vasculopathy | Morbilliform rash in trunk, acral cyanosis, ulcerated purpuric plaque | 68 | M | 1 |
| (12) | Recovery | Viral exanthema or urticariform dermatitis with discrete blood extravasation | Rash under breasts, back, and genital area | 65 | F | 1 |
| (13) | Recovery | Leukocytoclastic vasculitis affecting dermal vessels, extravasation of RBC, basal epidermal layer necrosis, dermal perivascular neutrophil infiltration, and fibrin deposition | Purple palpable papules. blisters on both her legs, feet, and toes, five days before pulmonary symptoms | 83 | F | 1 |
| (14) | Recovery | Perivascular mixed inflammatory infiltration of neutrophils and lymphocytes and extravasation of RBCs in upper dermis | Multiple red raised lesions symmetrically in extremities. | 22 | M | 1 |
| This case | Recovery | Leukocytoclastic vasculitis affecting dermal vessels | Purpuric rash on the trunk and lower extremities. | 21 | M | 1 |