1. Background
Nephrotic syndrome (NS) treatment in pediatrics is still a serious challenge. The current method of treatment is prednisone/prednisolone for months or weeks at the onset of presentations. These materials expose pediatrics to side effects and complications of steroid on growth, metabolism, and behavior (1). Moreover, 7.4 - 19.6% of cases are resistant to this agent as steroid therapy (2, 3). For those resistant and/or intolerant to corticosteroids, second-line immunosuppressive agents are used, which have additional complications with response rates of 20 - 50% (4). Cases who have refractory NS inevitably progress to end-stage renal disease (ESRD) (5). Another challenge is focal segmental glomerulosclerosis (FSGS), which is one of the most common histologic subtypes of pediatric NS with a 15 - 30% risk of recurrence in kidney transplant cases (6). Identifying additional therapeutic methods is very important.
Therapeutic effect of anti-allergy agents for NS has not been studied in different therapeutic agents. In some clinical studies, anti-inflammatory agents were frequently used for allergic rhinitis. In addition, anti-leukotrienes were used for asthma, and both were used for urticaria (7, 8). Montelukast provides some benefits for asthma (9). For atopic dermatitis, which is thought to be strongly related to NS, beneficial evidence was observed for cyclosporin A (10), which is a common immunotherapy for minimal change nephritic syndrome (MCNS). Therefore, this clinical trial study prospectively investigated montelukast as a novel and additional agent for pediatrics with NS.
2. Objectives
This study aimed to evaluate the effect of montelukast as an adjunct therapy of NS in pediatrics.
3. Methods
3.1. Setting and Populations
Patients with NS diagnosis admitted to Amir Kabir hospital, Arak, Iran, were considered as the study group. The patients were randomly divided into intervention (steroid + montelukast) (N = 25) and control (N = 25) groups.
3.2. Statistical Analysis
Quantitative data analysis was performed by independent sample t-test, and qualitative data analysis was performed by chi-square test. All analysis was done by statistical package for the social sciences (SPSS) software version 21, and P < 0.05 was considered as the significant value.
3.3. NS Diagnosis
NS, defined as protein excretion more than 40 mg/m2/h of body surface into urine and/or protein/creatinine ratio greater than 2-3 in the first-morning urine sample.
3.4. Selective Therapeutic Method and Response Types
Prednisolone 2 mg/kg (maximum dose 60 mg/kg/day) at 4 weeks was prescribed. After 4 weeks of treatment, the following were considered as classification of steroid response pattern.
3.5. Intervention and Control
In the intervention group, in addition to the usual treatment of NS, montelukast 5 mg/day was used as an adjunct to steroid therapy for one month. In the control group, selective therapeutic method (steroids) was used. Urine protein was measured to evaluate the renal status of patients and the status of NS.
3.6. Inclusion and Exclusion Criteria
This study included pediatrics with NS criteria diagnosis. Patients who did not refer to the center for treatment after one month and those with underlying and/or chronic diseases were excluded from the study.
3.7. Ethical Considerations
Ethical committee of Arak University of Medical Sciences, Iran, approved this study (ethical code: IR.ARAKMU.REC.1398.228; IRCT code: IRCT20130518013366N13).
4. Results
4.1. Demographic Characteristics
Mean ± SD of age in intervention and control groups was 7.26 ± 4.23 and 6.79 ± 3.91 years, respectively. In each group, 15 (60%) cases were male, and 10 cases (40%) were female. Based on statistical evaluation, there was no significant difference regarding age (P = 0.68) and gender (P = 1.0) in both groups (Table 1).
| Variables | Groups | Statistical Value | |
|---|---|---|---|
| Intervention | Control | ||
| Age, Mean ± SD | 7.26 ± 4.23 | 6.79 ± 3.91 | 0.68 |
| Gender, No. (%) | 1.0 | ||
| Male | 15 (60.0) | 15 (60.0) | |
| Female | 10 (40) | 10 (40) | |
4.2. Clinical Characteristics
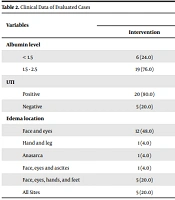
Albumin levels in 96% of control group and 76% of intervention group were 1.5-2.5 μg/dL (P = 0.042). Also, 80% of the control group and 84% of the intervention group did not have urinary tract infections (UTIs) (P = 0.713). The edema sites in 52% of control group and 48% of the intervention group was in the face and eyes (P = 0.636). Based on statistical evaluation, there was no significant difference between edema site and UTI, but albumin levels showed a significant difference (P = 0.042) (Table 2).
| Variables | Groups | Statistical Value | |
|---|---|---|---|
| Intervention | Control | ||
| Albumin level | 0.042 | ||
| < 1.5 | 6 (24.0) | 1 (4.0) | |
| 1.5 - 2.5 | 19 (76.0) | 24 (96.0) | |
| UTI | 0.71 | ||
| Positive | 20 (80.0) | 21 (84.0) | |
| Negative | 5 (20.0) | 4 (16.0) | |
| Edema location | 0.63 | ||
| Face and eyes | 12 (48.0) | 13 (52.0) | |
| Hand and leg | 1 (4.0) | 3 (12.0) | |
| Anasarca | 1 (4.0) | 0 (0.0) | |
| Face, eyes and ascites | 1 (4.0) | 1 (4.0) | |
| Face, eyes, hands, and feet | 5 (20.0) | 6 (24.0) | |
| All Sites | 5 (20.0) | 2 (8.0) | |
4.3. Therapeutic Response
In the control group, 33.33% of the children have frequent relapse change, therapeutic response in the control group in two evaluated time intervals, have been showed a statistically significant difference (P = 0.027). In the intervention group, 33.33% of the children who were corticosteroid dependent before the intervention and 71.85% of the children who were frequent relapse before intervention changed to responsive type; so, there was a statistically significant difference (P = 0.035) (Table 3).
| After Intervention | Statistical Value | ||||
|---|---|---|---|---|---|
| Steroid Response | Steroid Dependence | Frequent Relapse | Steroid Resistance | ||
| Control | 0.027 | ||||
| Steroid response | 7 (58.33) | 0 (0.0) | 2 (33.33) | 0 (0.0) | |
| Steroid dependence | 5 (41.67) | 1 (25.0) | 4 (66.67) | 2 (66.67) | |
| Frequent relapse | 0 (0.0) | 2 (50.0) | 1 (33.33) | 0 (0.0) | |
| Steroid resistance | 0 (0.0) | 1 (25.0) | 0 (0.0) 0 | 0 (0.0) | |
| Montelukast | 0.035 | ||||
| Steroid response | 7 (70.0) | 2 (33.33) | 6 (85.71) | 0 (0.0) | |
| Steroid dependence | 2 (20.0) | 2 (33.33) | 0 (0.0) | 1 (50.0) | |
| Frequent relapse | 0 (0.0) | 1 (16.67) | 1 (50.0) | 1 (14.29) | |
| Steroid resistance | 1 (10.0) | 1 (16.67) | 0 (0.0) | 0 (0.0) | |
5. Discussion
The treatment method for NS is still a challenging issue. The current therapeutic method is prednisolone/prednisone, with some complications on growth, metabolism, and behavior (1). Moreover, 7.4-19.6% of cases are resistant to this agent as steroid therapy (2, 3). Thus, identifying additional therapeutic method is very important. There are some clinical studies that mentioned anti-inflammatory agents are frequently used for allergic rhinitis; in addition, anti-leukotrienes drugs were used for asthma and urticarial (7, 8). So, we consider montelukast as an add-on therapeutic method for NS.
In present study, albumin levels in 95% of control group and 70% of intervention group were 1.5-2.5 μg/dL (P = 0.037). Also, 48% of the control group were corticosteroid dependent, and 60% of the intervention group responded to corticosteroids (P = 0.194). Therapeutic response in the control (P = 0.027) and intervention (P = 0.035) groups in two time intervals showed a statistically significant difference. Regarding the effect of montelukast on NS, Zedan et al. observed that the normal range of protein/creatinine ratio and diastolic blood pressure in the montelukast group were significantly higher than the control group (11); however, we did not assess blood pressure and protein to creatinine ratio in this study. Wang et al. evaluated the therapeutic effect of Ofatumumab on patients with NS and observed that out of four patients, three cases were cured, and one patient was partially cured (1); however, no complication was observed, and the cure recovery rate was higher than the present study. Esfehani et al. examined the long-term clinical outcome of 745 children with steroid-sensitive NS and observed that 9.2% of patients were responsive, while 15.8% were frequent relapse. At the last visit, 49.7% of patients were in remission, 32.5% were in recurrence, and 29% had chronic renal failure (CRF) (12), which was different from the results of present study. However, most studies have mentioned that anti-leukotriene agents can improve conditions and management of pediatrics with NS; further studies are required to confirm this issue.
5.1. Conclusions
Although recovery rate was higher in the intervention group, the difference was not statistically significant (P = 0.63). Further studies are needed to find best therapeutic methods for pediatrics with NS.