1. Background
Urinary tract infection (UTI), the most common bacterial infection of childhood, is associated with long-term and potentially irreversible complications such as renal scarring, hypertension, and end-stage renal disease (1). Early diagnosis, adequate and timely treatment, as well as extended follow-up can reduce these complications.
Factors including age, gender, and circumcision status in boys influence the epidemiology of UTI during childhood. During the first year of life, especially the first three months, boys are more susceptible to UTI, but thereafter, UTI occurs more frequently in girls (2). Up to the age of 7 years, at least one episode of UTI occurs in approximately 5% of girls and 2% of boys (3).
Although urine culture remains the gold standard for the diagnosis of UTI (4), various serum and urine biomarkers have been studied in this regard, including C-reactive protein (CRP), serum white blood cell (WBC) count, leukocyte esterase, procalcitonin, plasma neutrophil gelatinase-associated lipocalin, immunoglobulin A, xanthine oxidase, lactoferrin, interleukins, heparin-binding protein, myeloperoxidase, and many others (5, 6). Of note, despite the acceptable performance of some of these markers in the diagnosis of UTI, their utility is limited due to the cost and availability.
D-dimer is a fibrin degradation product reflecting the activation of the coagulation system (7). Moreover, as an acute phase reactant, D-dimer increases in inflammatory conditions with the stimulation of high levels of cytokines (8). However, the correlation between D-dimer levels and infections or inflammation have only been addressed in few studies (9).
2. Objectives
This study aimed to evaluate the role of plasma D-dimer as a marker of UTI in pediatric patients aged 1 month to 14 years.
3. Methods
3.1. Participants
This cross-sectional study included febrile children with symptoms of UTI admitted to Bandar Abbas Children's Hospital from October 1, 2017 to April 1, 2018. Inclusion criteria were age of 1 month to 14 years and positive urine culture (≥ 105 CFU/mL in bagged specimens, ≥ 104 CFU/mL in catheterized specimens, or ≥ 102 CFU/mL in suprapubic aspirations). Exclusion criteria were any underlying diseases, including liver or kidney conditions, history of recurrent UTIs, immune deficiency, coagulation disorders, drug history of antibiotics or anticoagulants, and presence of infection in other organs. Based on the study by Rodelo et al. (10), the sample size was calculated as at least 38 patients, with an expected correlation coefficient of 0.60, α = 0.05, and β = 0.02.
3.2. Study Design
Demographic features of all patients, including age and gender were recorded. All patients were visited and examined by a pediatric nephrologist. Body temperature was measured using a standard tympanic thermometer and fever was defined as > 38°C in children < 3 years, > 37.8°C in children 3 - 11 years, and > 37.6°C in children > 11 years. Accordingly, all children in the study were febrile. Urinalysis and urine culture were performed for all participants and those with a positive urine culture were included. Before the initiation of antibiotics, a random venous blood sample was collected from each participant. D-dimer was measured quantitatively using latex enhanced immunoturbidometric assay (Sclavo D-Dimer kit, Sclavo Diagnostics International, Italy) with Mindray-BS-800M Clinical Chemistry Analyzer (Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China). WBC count was determined using an automated blood cell counter. The percentages of neutrophils were also noted. Erythrocyte sedimentation rate (ESR) was measured using an ESR reader. CRP was measured quantitatively using Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran) and a biochemistry autoanalyzer (Technicon RA-1000, Technicon Instruments Corp. Terrytowen, NY, USA) with the immunoturbidity method.
3.3. Data Analysis
The Statistical Package for the Social Sciences (SPSS) software (version 25.0, Armonk, NY: IBM Corp., USA) was used for data analysis. Mean, standard deviation, frequency, and percentages were used to describe the results. Based on the results of Kolmogorov-Smirnov normality test, Spearman’s correlation test was used to evaluate the correlation of D-dimer with other markers, as well as all the markers with age. WBC count and neutrophil percentage were normally distributed in both genders; thus, the independent t-test was used to compare them between male and female participants. The Mann-Whitney test was used for the comparison of other markers by gender. P-values ≤ 0.05 were considered statistically significant.
4. Results
Out of 41 children (mean age: 5.50 ± 4.01 years) included in this study, 6 (14.6%) were male, and 35 (85.4%) were female. General characteristics of the study population are demonstrated in Table 1.
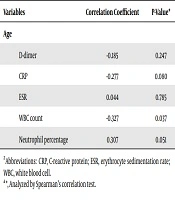
While plasma D-dimer level and WBC count were higher in females, CRP, ESR, and neutrophil percentage were higher in males; however, none of the gender differences were statistically significant (Table 2). Only the correlation between WBC count and age was significant (r = -0.327, P = 0.037). The inverse correlation of D-dimer with age was not statistically significant (r = -0.185, P = 0.247) (Table 3).
| Variables | Girls (N = 35) | Boys (N = 6) | P-Valueb |
|---|---|---|---|
| D-dimer, µg/L | 1645.78 ± 2985.68 | 625.59 ± 693.15 | 0.253† |
| CRP, mg/L | 10.31 ± 9.8 | 12.04 ± 15.33 | 0.447† |
| ESR, mm/h | 29.37 ± 27.19 | 33.50 ± 31.37 | 0.839† |
| WBC count, /µL | 9742.86 ± 2657.65 | 8883.33 ± 7124.16 | 0.781 |
| Neutrophil, % | 53.66 ± 22.30 | 60.17 ± 19.36 |
Comparison of D-Dimer and Other Markers by Gendera
| Variables | Correlation Coefficient | P-Valuea |
|---|---|---|
| Age | ||
| D-dimer | -0.185 | 0.247 |
| CRP | -0.277 | 0.080 |
| ESR | 0.044 | 0.785 |
| WBC count | -0.327 | 0.037 |
| Neutrophil percentage | 0.307 | 0.051 |
Correlation of D-Dimer and Other Markers with Age
Plasma D-dimer level was significantly correlated with ESR (r = 0.647, P = 0.026) and CRP (r = 0.525, P = 0.001); however, there was no significant association between D-dimer with WBC count and neutrophil percentage (P > 0.05) (Table 4).
| Variable | Correlation Coefficient | P-Valuea |
|---|---|---|
| D-dimer | ||
| CRP | 0.525 | 0.001 |
| ESR | 0.647 | 0.026 |
| WBC count | 0.236 | 0.137 |
| Neutrophil percentage | 0.201 | 0.208 |
Correlation of D-Dimer with Other Markers
5. Discussion
In the current study, we found a significant positive correlation between plasma D-dimer level with ESR and CRP in children. Since ESR and CRP are confirmed indicators of inflammation, this shows that D-dimer can also be indicative of inflammation. It has been shown that D-dimer can be used for the assessment of coagulation disorders, as well as the presence and severity of acute infection in children. Also, it has been reported that along with other inflammatory markers, D-dimer can be used as an inflammatory marker in infants with febrile UTI (9).
Since D-dimer is the final product of fibrin degradation and reflects the activation of the coagulation cascade, its levels can increase in conditions involving thrombosis, such as venous thromboembolism, disseminated intravascular coagulation (DIC), infection, inflammation, and even stroke or ischemic heart disease (7, 11). Indeed, D-dimer has long been a part of the criteria for the diagnosis of pulmonary thromboembolism (12). Sharma et al. recommended the measurement of D-dimer in children with sepsis for the early prediction of DIC (13). Also, D-dimer has been proposed as an appropriate prognostic marker in pediatric acute appendicitis (14). Nonetheless, only one previous study conducted by Lee et al. (9) reported its significance as an inflammatory marker of UTI; in their study, D-dimer was superior to other markers, including serum WBC count and ESR, but was inferior to CRP in predicting upper UTI (9). D-dimer has also been proposed as a strong prognostic factor in patients with suspected infection or sepsis (10).
Another finding of the current study was that plasma D-dimer level was not influenced by age or gender. This adds to the applicability of this test for the diagnosis of UTI in children. However, whether plasma D-dimer is sensitive and specific enough in this regard to dispense us with other laboratory investigations is debatable and requires further studies.
D-dimer is a simple, relatively inexpensive and available test which shows the activity of the coagulation system and potentially the severity of host response to infection. Nonetheless, D-dimer results are dependent on the sensitivity and specificity of the measurement kits used for this purpose. Thus, change in D-dimer levels can be more useful than its absolute values. Moreover, renal dysfunction and the resultant decrease in D-dimer excretion may not be the only reason for increased D-dimer levels, and activation of the coagulation system in such patients can be another cause (15, 16). That is why we excluded patients with underlying kidney diseases.
Other biomarkers such as mean platelet volume, procalcitonin, neutrophil gelatinase-associated lipocalin, and many more have been used for the diagnosis of UTI, especially in the pediatric population (17-20). Future studies are required to compare the applicability of these biomarkers with D-dimer.
One limitation of the current study was that we could not assess the sensitivity and specificity of D-dimer for the diagnosis of UTI since there were no controls such as children with afebrile UTI or febrile illness due to conditions other than UTI. Although our findings suggest that D-dimer can be used as an alternative for ESR or CRP in case of limited resources, it is not clear what real value measurement of D-dimer adds to the evaluation or management of patients with suspected or known UTI. Therefore, in future studies, a case-control design with a larger sample size would be preferred.
5.1. Conclusions
The results of the current study demonstrated that D-dimer is a useful marker for the diagnosis of UTI in children, regardless of age and gender. However, its diagnostic performance has to be further determined.