1. Background
Idiopathic membranous nephropathy (IMN) is an organ-specific autoimmune disease. The most common antigens identified are phospholipase A2 receptor M (PLA2R) (1, 2) and thrombospondin type-1 domain 7A (THSD7A) (3). In a retrospective study conducted in Senegal between 2011 and 2015, membranous nephropathy (MN) accounted for approximately 10.68% of glomerular lesions (third glomerulopathy) (4). The B cell has been shown to be implicated in the pathogenesis of IMN (5). Hence, the interest of Rituximab, a murine/human monoclonal antibody, directed against the B cell CD20 antigen. Rituximab at a dose of 375 mg/m2/week for four weeks in eight patients with IMN was associated with the achievement of remission in two-thirds of the patients at six months and also at 12 months (6). The GEMRITUX study demonstrated the efficacy of Rituximab in achieving durable remission (7). A more recent study showed that in addition to these satisfactory results, it partially improved renal function in patients with a glomerular filtration rate (GFR) less than 30 mL/min/1.73 m2 (8).
In sub-Saharan Africa, particularly in Senegal, most therapeutic protocols, including immunosuppressants drugs, are difficult to implement for several reasons, notably due to the low socio-economic level and the remoteness of the treatment centers.
Thus, we formulated the following research question “What is the impact of Rituximab in the treatment of patients with IMN in Senegal?”
2. Objectives
The main objectives of this study were to evaluate the effectiveness of Rituximab in the management of IMN based on the following criteria: (I) Biological remission at three months (M3) and at six months (M6); (II) evolution of the average PU24, serum albumin, and serum creatinine levels at M3 and M6; (III) and side effects.
3. Methods
This retrospective observational study had a descriptive and analytical design. The study was approved by the research ethics committee of the Universite Cheikh Anta Diop (UCAD), Senegal and registered under the reference number CER/UCAD/AD/MsN/06/2020.
Patients with IMN, nephrotic syndrome, and positive anti-PLA2R antibodies who received at least two courses of Rituximab after six months of conservative treatment without remission were included. Patients who had immunosuppressive therapy within six months before inclusion and those with unexplainable records were excluded.
Sociodemographic (gender and age), clinical (edema and blood pressure), paraclinical (24hPU, serum albumin, and serum creatinine levels), therapeutic and evolutionary parameters (complete or partial remission at three and six months) were collected using a pre-established collection form the patients’ medical records. Telephone calls were made to collect some of the missing data from the records.
Complete remission was defined as PU < 0.3 g/24 h with serum albumin > 35 g/L and normal renal function. Partial remission was defined as PU < 3.5 g/24 h and less than 50% of baseline PU associated with a decline or stabilization of renal function.
The collected data were entered into Excel and analyzed using the Statistical Package for Social Sciences (SPSS) V21 software.
In the descriptive analysis, qualitative parameters were described by their proportion (%). Quantitative parameters were described by their position (mean or median) and dispersion (standard deviation or extremes) parameters. Means PU, serum albumin, and serum creatinine levels were compared between M0, M3, and M6 using student t-test. A P-value < 0.05 was considered as a statistical significance.
4. Results
Six patients were included in the study. One patient was lost to follow-up after the administration of two doses of Rituximab. Thus, five patients (four males and one female) were analyzed (P1, P2, P3, P4, and P5). Edema was present in all patients, and three patients had anasarca state. Other patient characteristics at inclusion are reported in Table 1.
| Parameters | P1 | P2 | P3 | P4 | P5 | Means ± SD |
|---|---|---|---|---|---|---|
| Age, y | 29 | 39 | 75 | 18 | 60 | 44.20 ± 23.14 |
| SBP, mmHg | 100 | 140 | 170 | 110 | 130 | 130 ± 27.38 |
| DBP, mmHg | 80 | 85 | 95 | 60 | 90 | 82 ± 13.5 |
| PLA2R, IU | 10 | 100 | 800 | 10 | 100 | 204 ± 336.19 |
| PU24, g/24 h | 5.37 | 8.27 | 7.00 | 6,10 | 5.99 | 6.54 ± 1.13 |
| Serum albumin, g/L | 19.50 | 14.80 | 8.00 | 14.00 | 21.50 | 15.56 ± 5.27 |
| Serum creatinine, mg/L | 22.10 | 13.00 | 24.00 | 6.00 | 21.40 | 17.3 ± 7.6 |
Abbreviations: DBP, diastolic blood pressure; P, patient; PU, proteinuria; SBP, systolic blood pressure; SD, standard deviation.
One patient had chronic contact eczema, and vagotomy for peptic ulcer disease was reported in one patient.
All patients had IMN type 2. The associated histological signs were: acute tubular necrosis + interstitial fibrosis (2/5), interstitial infiltration (1/5), tubular atrophy + interstitial fibrosis (1/5), and extra capillary glomerulonephritis (1/5).
All patients had received a biosimilar of Rituximab at a dose of 1 g at day 0 and 1 g at day 14. One patient (P3) had received a third dose at M6. All patients had premedication: Paracetamol (5/5), Methylprednisolone (5/5), Hydrocortisone (4/5), Antiemetic (2/5). One patient (P5) had received a modified Ponticelli protocol prior to Rituximab administration. Renin angiotensin system blockers were used in all patients: ACE inhibitors (5/5) and ARBs in place of ACE inhibitors (3/5). All patients had received diuretics: Furosemide (5/5), Anti-aldosterone (3/5), and Hydrochlorothiazide (1/5). Other treatments were: Acenocoumarol (3/5), Rivaroxaban (1/5), Enoxaparin (1/5), Statins (2/5), Cotrimoxazol + Thrimetroprine (5/5), and Valganciclovir (5/5). P4 had presented seizures and pruritus just after the first dose of Rituximab. P5 had presented rectal bleeding due to anti-vitamin K overdose.
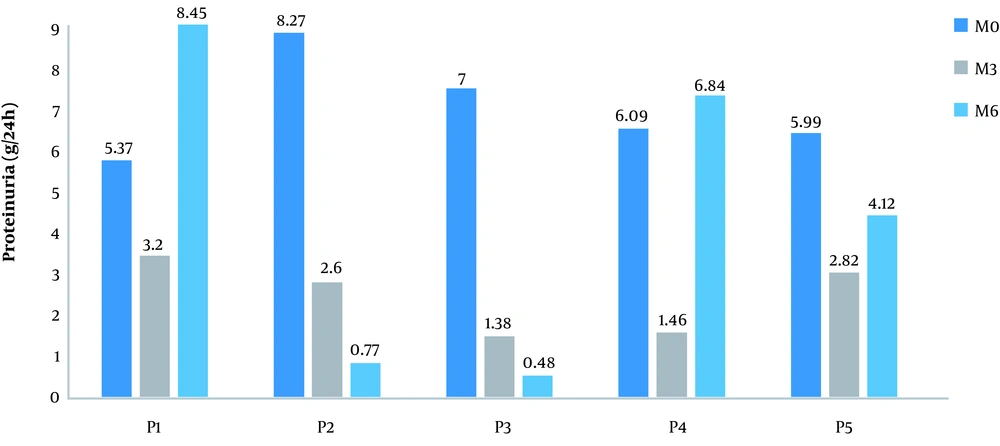
Partial remission was noted in three patients at M3 (P2, P4, and P5) and in one patient (P2) at M6. No complete remission was noted. The mean PU had decreased significantly (P < 0.001) from M0 to M3 (Table 2).
| Parameters | M0 | M3 | M6 |
|---|---|---|---|
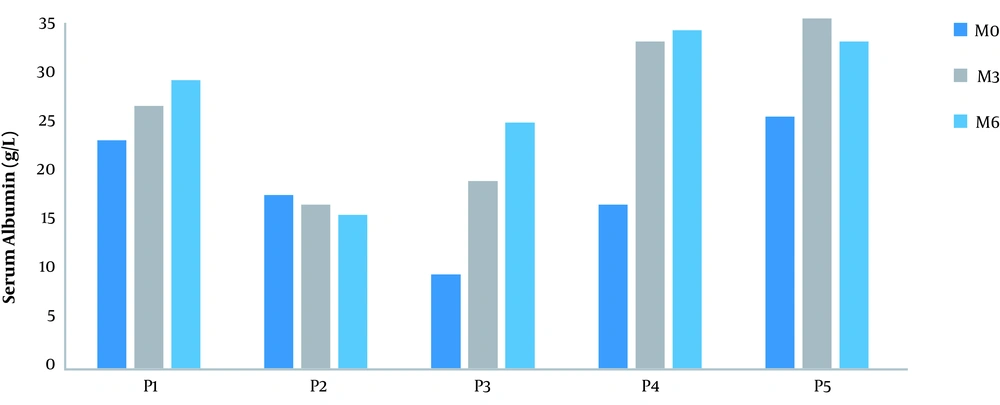
| Serum albumin, g/L | 15.56 ± 5.27 | 22.06 ± 7.02 | 23.10 ± 6.39 |
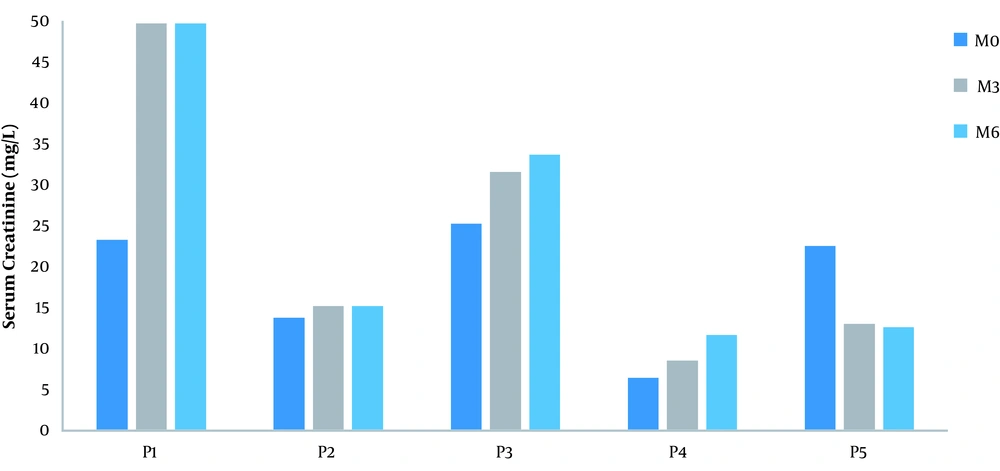
| Serum creatinine, mg/L | 17.3 ± 7.60 | 16.76 ± 11.76 | 23.32 ± 15.90 |
| Proteinuria, g/24 h | 6.54 ± 1.13 | 2.29 ± 0.82a | 4.13 ± 3.56 |
aSignificant decrease of proteinuria from M0 to M3 (P < 0.001).
An increase in PU at M6 was noted in three patients (Figure 1).
An increase of approximately 41.77% at M3 and 48.45% at M6 in the baseline serum albumin value was noted (Figure 2). The serum creatinine was stable in three patients from M0 to M6 (Figure 3). The median anti-PLA2R antibody titer at diagnosis was 100 IU/L with extremes of 10 and 800 IU/L.
Serum creatinine level of each patient from M0 to M3 and M6 after Rituximab administration. Kidney function was preserved in one patient prior to Rituximab administration. It was normal in two patients at M3 and M6. A decrease or stabilization of kidney function was noted in three patients at M3 and M6 (P2, P4, and P5).
The anti-PLA2R antibody titer was measured at M3 in P3, which was greater than 800 IU. The CD19 lymphocytes level was measured in P2 at M6, which was 63/mm3 (3% of circulating lymphocytes).
5. Discussion
As far as the researchers investigated, there is currently no data on the evaluation of the efficacy of Rituximab in the management of IMN in sub-Saharan Africa. Thus, we felt it was necessary to conduct this study to evaluate its efficacy.
Several previous studies have demonstrated the efficacy of Rituximab, leading to several recommendations for its use; however, most of the studies had been conducted outside the African continent. This is probably due to the high cost of Rituximab and the problems of access to biopsy, immunofluorescence, and anti-PLA2R antibody titers. Indeed, in the absence of universal health coverage in most sub-Saharan African countries, this molecule is practically not used in the management of IMN.
The first result of this work is the absence of complete remission in patients despite the high dose protocol used. However, three patients were in partial remission at M3, and one patient had maintained this remission at M6. A significant decrease of more than 50% in mean PU at M3 after Rituximab administration (P < 0.001) was noted as reported in the literature (6, 9). This remission rate at M6 was low compared to other studies using Rituximab (1, 6, 7, 10), alkylating agents (11-14), or anticalcineurin (15, 16). With this result, the main question is whether Rituximab should be used as first-line therapy in the management of IMN in sub-Saharan Africa? The cost of two doses of Rituximab in Dakar is 1819.64 USD, which is extremely expensive for the majority of the population in sub-Saharan Africa. But Cyclophosphamide is more accessible, it has the same effectiveness regarding remission rate, and it does not cause more side effects (17).
The second finding is the difficulty of biological and immunological follow-up of patients treated with Rituximab. The CD19 lymphocytes level was measured in a single patient during the follow-up l. Thus, neither the immunological remission nor the impregnation of Rituximab could be studied in the patients. These follow-up difficulties are linked to the problems of financial means in a context where health coverage is lacking. In the literature, immunological remission by Rituximab precedes clinical remission (1, 11, 18). The anti-PLA2R antibody titer is currently the cornerstone of follow-up in IMN. The higher the titer, the lower the remission rate (7, 9).
The third finding is the increase in mean serum albumin levels by 41.77% and 48.45% from baseline at M3 and M6, respectively. A smaller increase is reported in the literature (6, 7). However, none of the patients had a normalized serum albumin level at M6. Serum creatinine levels remained stable until M6 after Rituximab administration. These results were in accordance with those of the Remuzzi et al. (6).
The fourth result is the tolerance of Rituximab in patients as reported in the literature (19). Indeed, only one patient presented seizures and generalized pruritus after the first dose. This reaction was absent after subsequent doses.
The main limitations of this study include the small sample size, the retrospective nature that may produce bias, and the problems of follow-up.
The main difficulty encountered in this study was poor medical record keeping. Indeed, several paraclinical data were not reported in the records.