1. Background
Urinary tract infection (UTI) is common in children that may lead to serious morbidity as a result of an inflammatory response (1). The UTI is divided into three forms, including pyelonephritis (PN), cystitis, and asymptomatic bacteriuria. The PN is characterized by non-specific symptoms, including fever and one or more of the following manifestations: Flank pain, back or abdominal pain, nausea, vomiting, and occasionally diarrhea (2). Many patients with PN show some electrolyte disturbances, including hyponatremia and hyperkalemia (2-4).
Hyponatremia is a prevalent electrolyte disorder in hospitalized patients that results from a disruption of balance between water and body sodium levels and is defined by a serum sodium level of less than 135 mEq/L (5, 6). Hyponatremia may be noticed in PN. Although hyponatremia is usually mild, it can sometimes be severe and lead to severe morbidity even in patients with UTI (7). Metabolic acidosis and hyperkalemia are other abnormalities reported in these patients (8).
Although dimercaptosuccinic acid (DMSA) scan is the most sensitive test for diagnosis of acute kidney damage following UTI, it appears to be of little benefit (9) if performed soon after the first UTI. It identifies acute kidney parenchymal injury; however, most acute changes resolve over time regardless of whether antibiotic prophylaxis is used (10). There is little availability of DMSA scans, and these are expensive for families. It is associated with high radiation exposure, which may be carcinogenic (9, 11). On the basis of these reasons, we need some simpler, more secure, cheaper, and less burdensome tools to investigate the severity of renal parenchymal involvement in children with clinical PN.
2. Objectives
Therefore, the present study aimed to investigate the relationship between sodium and potassium changes and urine SG with renal involvement as documented by DMSA scan in children with febrile UTI admitted to Taleghani Hospital in Gorgan.
3. Methods
This is a retrospective cohort study, involving 158 children with febrile UTI admitted to Taleghani Hospital from 2018 to 2019. Their documents in hospital were analyzed. They were divided into two groups with positive or negative defects on DMSA scan. The inclusion criteria were all children aged two months to 14 years with febrile UTI. The exclusion criteria were afebrile children and those with chronic disease of renal or non-renal etiology, and also those with a coincident infectious disease other than UTI. Clinical data such as age, sex, and duration of fever were recorded. Laboratory data included complete blood count, ESR, CRP, serum concentrations of sodium, potassium, and urine analysis (UA). Imaging studies included renal ultrasonography (USG), DMSA scan, and VCUG. Urine was collected using a urine bag, catheterization, and suprapubic methods for infants. Midstream urine culture was used for toilet trained children. The urinary and serum tests were performed at the time of admission to the hospital before administration of antibiotics or fluids.
3.1. Statistical Method
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 18. Data were analyzed using independent t-test with normal distribution of variables, otherwise chi-square test was used. The statistical significance level of the study was considered 0.05.
3.2. Definitions
Fever was defined as a body temperature ≥ 38°C. Hyponatremia and hyperkalemia were considered when the serum sodium concentration was < 135 mEq/L and serum potassium > 5.5 mEq/L. The UTI was diagnosed in symptomatic patients with pyuria and positive urine culture. The urine culture was considered positive when there was growth of a single pathogen with a colony count ≥ 5 × 104 CFU/mL in suprapubic or catheter sample and ≥ 105 in midstream sample. In the bag sample, UTI was diagnosed when the child was symptomatic and had pyuria, as well as a positive culture with a colony count ≥ 105 of a single organism.
4. Results
Among the 158 children, 78 patients (49.4%) had normal DMSA scan results, and 80 patients (50.6%) had an abnormal result. Therefore, renal cortical involvement was present in about half of the febrile UTI cases in this study. The patients were divided into two groups; group one with normal and the other (group 2) with abnormal DMSA scan results. There was no difference between the number of boys and girls in the two groups studied (P = 0.709) (Table 1).
The mean age in month was not different between the two groups (P = 0.287) (Table 2).
| Variables | DMSA | P-Value | |
|---|---|---|---|
| Normal (Mean ± SD) | Abnormal (Mean ± SD) | ||
| Age (mon) | 27.12 ± 32.46 | 28.93 ± 30.10 | 0.287 |
| ESR (mm/h) | 45.38 ± 33.44 | 58.30 ± 34.22 | 0.015 |
| Sodium level (mEq/L) | 140.41 ± 3.33 | 139.49 ± 3.08 | 0.058 |
| Potassium level (mEq/L) | 4.53 ± 0.58 | 4.09 ± 0.54 | 0.484 |
| Urine SG | 1013 ± 7.73 | 1013 ± 6.72 | 0.962 |
| WBC count (count per mm3) | 13016.67 ± 5919.75 | 13301.13 ± 6517.14 | 0.768 |
| Duration of fever (day) | 5.04 ± 4.86 | 7.34 ± 7.16 | 0.084 |
Mean and SD of Variables by DMSA Scan Results
In patients with positive renal cortical defects on DMSA scan (group 2), ESR was significantly higher than the first group (58.3 vs 45.38 P = 0.015) (Table 2). The mean serum Na level in the second group was lower than in the first group; however, it was only slightly significant (139.4 vs 140.41, P = 0.058) (Table 2). The two groups showed no difference with respect to serum potassium, urine specific gravity, WBC count, and the duration of fever (P > 0.05) (Table 2).
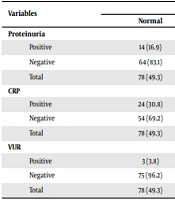
The frequency distribution of proteinuria according to DMSA scan was not different between the two groups (P = 0.836) (Table 3) there was a significant difference of positive CRP value between the first and the second group (30.8 vs 50%, P = 0.016). The frequency distribution of vesicoureteral reflux (VUR) was quite higher in those with a positive DMSA scan than in those with a normal scan (15 vs 3.8%, P = 0.027) (Table 3).
| Variables | DMSA | Total | P-Value | |
|---|---|---|---|---|
| Normal | Abnormal | |||
| Proteinuria | 0.709 | |||
| Positive | 14 (16.9) | 15 (18.8) | 29 (17.8) | |
| Negative | 64 (83.1) | 65 (81.3) | 129 (82.2) | |
| Total | 78 (49.3) | 80 (50.7) | 158 (100) | |
| CRP | 0.016 | |||
| Positive | 24 (30.8) | 40 (50) | 64 (40.5) | |
| Negative | 54 (69.2) | 40 (50) | 94 (59.5) | |
| Total | 78 (49.3) | 80 (50.7) | 158 (100) | |
| VUR | 0.027 | |||
| Positive | 3 (3.8) | 12 (15) | 15 (9.5) | |
| Negative | 75 (96.2) | 68 (85) | 143 (90.5) | |
| Total | 78 (49.3) | 80 (50.7) | 158 (100) | |
The Results of Proteinuria, CRP, VUR According to DMSA Scan a
5. Discussion
The differentiation between acute pyelonephritis and cystitis is sometimes very challenging, especially in small children and infants who show non-specific symptoms. The purpose of this study is to investigate the changes in electrolytes and inflammatory markers with febrile UTI in children and to correlate these changes with renal parenchymal involvement as is evidenced by positive DMSA scan. These changes may help us to predict a more severe parenchymal involvement in order to treat these patients with a better strategy.
Moreover, ESR was higher in children with renal cortical defects on DMSA scan than those with normal scans (58.3 vs 45.38 P = 0.05). This finding is observed in other studies (2, 12). With regard to the frequency distribution of CRP, there was a significant difference of positive CRP value between the first and the second group (P = 0.016). We found no difference in WBC count between the two groups (P = 0.768), which was contrary to Park et al.’s study (2). The main drawback of these markers is that they are non-specific and can be elevated in other infections.
Pyelonephritis may cause pseudohypoaldosteronism as a result of renal tubular dysfunction and consequently, changes in serum sodium and potassium (13) and also may result in an increase in inflammatory markers like ESR and CRP, which are not observed in cystitis. These changes may help us differentiate PN from cystitis and predict a more severe renal parenchymal involvement (2, 4, 7, 12).
Decreased serum sodium level in PN is reported in some studies (2, 3, 7). This finding may occur as a result of different mechanisms. The first mechanism is secondary pseudohypoaldosteronism as a result of pyelonephritis. This results in the inability of tubules to respond to aldosterone due to severe tubular inflammation and a high intrarenal pressure (8, 13, 14). Second increased secretion of ADH may be induced by inflammatory cytokines such as IL-18, IL-6, and TNF-α as was proposed by Watanabe et al. (15) and Shin et al. (16). The increased ADH secretion may contribute to hyponatremia in inflammatory diseases. The release of inflammatory mediators could induce hyponatremia by a mechanism other than ADH secretion, that is a reduction in the expression and inhibition of the function of epithelial sodium channel and/or sodium/potassium adenosine triphosphates (ATPase) at the basolateral membrane of renal epithelial cells (15). It was shown that electrolyte disturbances are not encountered only in PN; in fact, fever and infections can produce the same abnormalities due to inflammatory mediators and the release of cytokines (17). According to our study, serum sodium levels were lower in children with PN compared with the first group, but the difference was not significant (139.49 vs 140.41, P = 0.058), maybe studies with the inclusion of a larger number of patients may better elucidate the difference. There was no difference in serum potassium level between the two groups (P = 0.484). There was also no difference in serum potassium level in the two groups of infants less than one-year-old (P = 0.196). Similar to our study, Park et al. also found no difference in potassium level between the two groups (2), but other studies have demonstrated a higher serum potassium level in pyelonephritis (12, 13). This difference may be due to inclusion of young infants with tubular immaturity in these studies.
The frequency distribution of proteinuria according to DMSA scan was not different between the two groups. The presence of tubular proteinuria as a marker to distinguish between upper and lower UTIs in children has been studied by many authors (18, 19). However, patients with lower UTI also showed proteinuria (19). It seems that urinary protein to creatinine ratio is not a relevant factor for predicting PN in children with UTI. The frequency distribution of VUR was quite higher in those with a positive DMSA scan than in those with a normal scan. This finding has been reported in other studies (20, 21). Reflux itself may be a predisposing cause of renal uptake defect and also scar formation (20, 21). We found no difference in WBC count (P = 0.768) and duration of fever (P = 0.084) between the two groups, which was contrary to Park et al.’s study (2).
5.1. Conclusion
We suggest that increased ESR, positive CRP, and the presence of reflux can predict renal parenchymal involvement in children with febrile UTI as evidenced by a positive finding on DMSA scan and the presence of hyponatremia has a little predictive value in this regard.