1. Background
The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which emerged in Wuhan city, Hubei province, China, is the third installment in the coronaviruses causing an outbreak in the past couple of decades (1). This virus, now best associated with the term coronavirus disease 2019 (COVID-19), has infected millions and culminated in the death of tens of thousands of patients all over the globe (2). The clinical spectrum of the disease varies from asymptomatic carriers to patients with multi-organ failure, acute respiratory distress syndrome (ARDS), and death. In a systematic review of studies with 660 patients, 32.8% presented with respiratory signs and symptoms, 6.2% were in shock, 7.4% had acute kidney injury (AKI), and 20.3% required ICU care (3). Most patients with mild disease experience fever, sore throat, dry or productive coughs, mild dyspnea, fatigue, myalgia, headache, anosmia, GI upset, cutaneous rash, and conjunctivitis (4). Severe lymphocytopenia, significantly elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), thrombocytopenia, derangements in coagulation tests, and elevated D-dimer levels are predictors of severe disease and poor prognosis (5). Besides, AKI, proteinuria, and hematuria are seen more commonly among ICU patients as negative prognostic factors with stepwise increments in hazard ratios for in-hospital deaths associated with the intensity of these complications (6, 7). The histopathological studies on patients with AKI reported collapsing glomerulopathy in at least three patients (8). Electron microscopy of specimens from 26 deceased patients demonstrated the infection of tubular epithelium and podocytes with viral particles (9).
On the other hand, patients with end-stage renal disease (ESRD) are at a higher risk of contracting the virus from hemodialysis centers and their routine visits to medical centers and are thought to be at increased risk of death because of older age and multiple co-morbidities such as diabetes and hypertension (10, 11). According to a study from a hemodialysis center in Wuhan, ESRD patients on intermittent or maintenance hemodialysis (MHD) were more susceptible to COVID-19 infection. However, progression to severe pneumonia or ARDS was rare compared to the healthy population. This finding was in contrast to what clinicians had expected. Although the mortality rate of COVID-19 in MHD patients was 31%, only 20% of the patients who succumbed to the disease died of respiratory failure. The authors concluded that these patients reduced inflammatory cytokine levels played a protective role against ARDS development (12). Although mortality rates are higher in this group of patients, lung failure seems not to be the main reason. In this study, we focused on ESRD patients infected with COVID-19, documented their clinical signs and symptoms, and evaluated chest CT findings and their evolution among ESRD patients on MHD in a university-based hemodialysis center.
2. Methods
2.1. Patients
The present retrospective study was conducted amongst patients presenting to a tertiary health care system with signs and symptoms of COVID-19 infection between January 2020 and July 2020. This health care system served four million people and had served approximately 12,000 COVID-19 patients. All ESRD patients with positive polymerase chain reaction tests for COVID-19 were evaluated for inclusion, and those who underwent chronic dialysis were recruited in the study. Exclusion criteria consisted of those patients with a history of renal transplantation, acute on chronic renal failure, and those who were not willing to participate in the study.
2.2. Acquisition of Clinical Data and Laboratory Findings
All laboratory test results and CT imaging findings were collected using the health information system of the institution where the study was performed. All clinical data of the patients were extracted either directly from clinical records or indirectly via phone calls from patients. All of the data gathered were entered into pre-existing checklists. Laboratory tests were performed via commercially available kits, and the molecular assay for COVID-19 infection was done by Taqman® Premix TAKARA kits (TaKaRa, Dalian, China). Specimens were collected from the nasopharynx and oropharynx and were processed and transferred to the lab, based on the World Health Organization guidelines.
2.3. Imaging Protocol
Imaging was performed based on national guidelines, which were in line with the World Health Organization guidelines (13). Imaging was done after consultation with infectious disease specialists. Imaging was done either on the day of admission or the day before admission by Siemens SOMATOM (Hannover, Germany) and Toshiba Alexion (Tokyo, Japan) scanners based on technical specifications mentioned in earlier studies (14, 15). The interpretation of imaging findings was made without prior knowledge of RT-PCR results.
2.4. Statistical Analysis
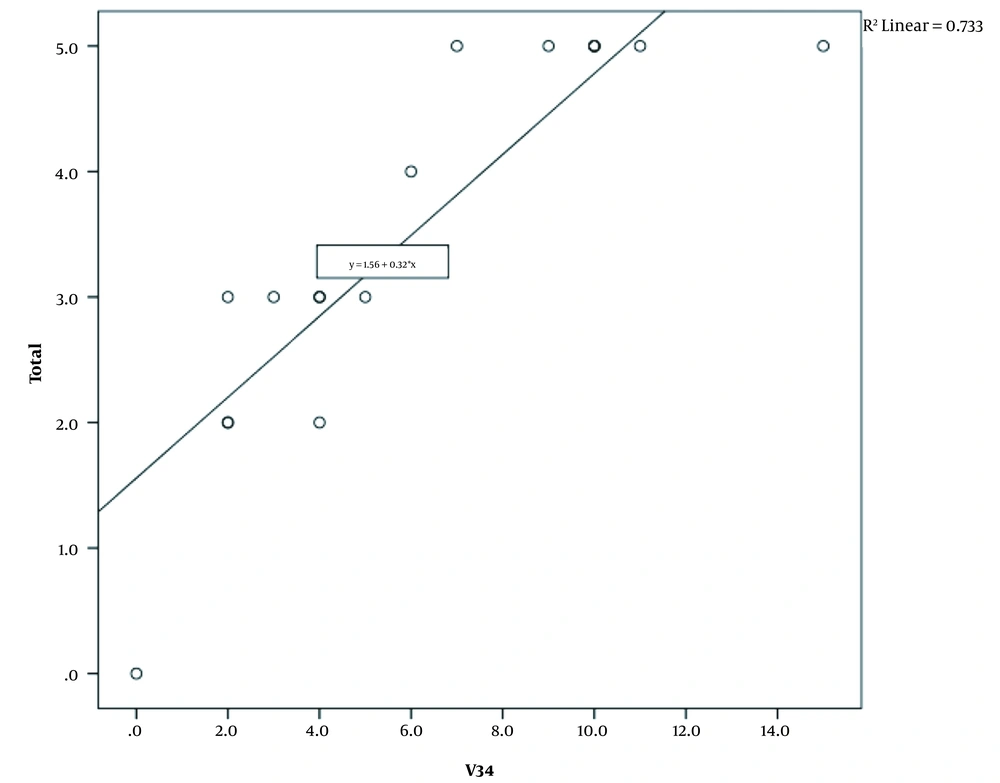
Statistical analysis was done by SPSS (version 23.0.0). Quantitative data were represented as mean ± standard deviation, and qualitative data as frequency and percentage. A scatter plot diagram was used to illustrate the relationship between the lung score and the number of involved lung lobes, and the trend line was shown on the diagram.
2.5. Ethical Considerations
The local ethics committee of Tabriz University of Medical Sciences approved the present study (Code: IR.TBZMED.REC.1398.1275). Informed consent was obtained from all patients before inclusion.
3. Results
A total of 17 patients were included in the present study. Six patients were females, and 11 were males. The mean age of the patients was 62.29 ± 15.6 years (22 - 82). The most common underlying condition was chronic hypertension. Other causes are shown in Table 1. Clinical signs and symptoms are also presented in Table 1. As seen, coughs were the most commonly reported clinical symptom.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration on dialysis (y) | 1 | 3 | 4 | 4 | 3 | 6 | 4 | 4 | 4 | 3 | 1 | 3 | 4 | 2 | 3 | 2 | 3 |
| Sex | F | M | M | M | M | M | M | M | F | F | M | M | M | F | F | F | M |
| Age | 22 | 64 | 70 | 76 | 72 | 82 | 65 | 66 | 81 | 72 | 34 | 61 | 69 | 65 | 50 | 56 | 54 |
| Diabetes | - | + | + | + | - | - | + | + | - | - | - | + | - | + | + | + | + |
| Hypertension | + | + | + | + | + | + | - | + | + | + | + | + | + | - | + | - | + |
| Glomerulonephritis | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - |
| Died | - | + | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - |
| Clinical signs and symptoms | |||||||||||||||||
| Fever | + | + | - | + | - | - | - | + | - | + | + | - | - | - | + | - | - |
| Coughs | + | + | + | - | + | + | - | + | + | - | - | + | + | - | + | - | + |
| Dyspnea | + | + | + | - | - | + | + | - | - | - | + | + | + | + | + | + | + |
| Malaise | - | + | - | + | - | - | + | - | - | - | - | - | - | - | + | - | - |
| Myalgia | + | + | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - |
| Diarrhea | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| Sore throat | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - |
| Anosmia | - | - | - | - | + | + | - | - | - | + | + | - | - | - | - | - | - |
| Other signs | - | + | - | - | + | - | - | - | - | - | - | + | - | + | - | - | + |
Laboratory results of the patients are presented in Table 2. Six patients had lymphocytosis, and eight patients had lymphopenia. Seven patients had elevated LDH levels, and two patients had elevated liver enzyme levels. The imaging findings of the patients are presented in Table 2. As shown, the most common imaging sign was ground-glass opacities, which were seen in 16 patients (94.1%). The most common involvement pattern was bilateral and peripheral involvement (13 patients; 76.5%). Posterior portions of the lungs were involved in 14 (82.4%) patients. The mean number of lung lobes involved was 3.5 ± 1.5 (0 - 5). The mean lung score was 6.12 ± 4.1 (0 - 15). The right lower lobe was the most common lobe involved, accounting for 16 (94.1%) patients. Table 3 presents the imaging findings of patients included in the study.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 12100 | 6200 | 8500 | 6700 | 4900 | 4100 | 19500 | 15500 | 20400 | 7400 | 1100 | 24400 | 12700 | 7000 | 5700 | 5000 | 5800 |
| Lymph | 2650 | 688 | 646 | 1842 | 1260 | 492 | 2106 | 1271 | 856 | 2715 | 451 | 488 | 457.2 | 1960 | 712.5 | 1295 | 1450 |
| Hb | 8.2 | 14.7 | 9.1 | 10.5 | 10 | 7.3 | 11.1 | 11.6 | 10 | 17 | 10.1 | 8.9 | 9.8 | 11.9 | 9.3 | 9.4 | 10.9 |
| Plt | 463000 | 101000 | 194000 | 164000 | 90000 | 112000 | 439000 | 394000 | 187000 | 199000 | 131000 | 202000 | 358000 | 156000 | 105000 | 177000 | 214000 |
| ESR (1) | 78 | 36 | 122 | 58 | 41 | 37 | 92 | 113 | 110 | 32 | 56 | 78 | 73 | 29 | 88 | 33 | 32 |
| CRP | 2+ | 3+ | 1+ | 4+ | _ | 2+ | 1+ | 2+ | 2+ | _ | _ | 3+ | 1+ | 1+ | 1+ | _ | 1+ |
| LDH | 486 | 686 | 601 | 315 | 391 | 1183 | 489 | 754 | 247 | 323 | 773 | 641 | 621 | 843 | 676 | 349 | 545 |
| AST | 22 | 29 | 42 | 16 | 260 | 104 | 16 | 56 | 26 | _ | 78 | 56 | 112 | 29 | 21 | 26 | 10 |
| ALT | 9 | 14 | 23 | 21 | 103 | 73 | 11 | 43 | 10 | _ | 65 | 45 | 65 | 14 | 7 | 34 | 9 |
| ALK.P | _ | 955 | _ | _ | 591 | _ | 172 | _ | 162 | _ | _ | _ | _ | 335 | _ | 184 | 191 |
| Na | 141 | 130 | 133 | 137 | 135 | 131 | 125 | 134 | 142 | 141 | 138 | 135 | 138 | 134 | 131 | 138 | 138 |
| K | 4 | 7 | 6.4 | 4.5 | 3.6 | 6 | 5.8 | 5 | 4.4 | 3.9 | 5 | 4.4 | 5.1 | 4.3 | 3.9 | 5.5 | 4 |
| Ca | _ | 9.3 | _ | 8.9 | 7.4 | _ | 6.1 | _ | 9.9 | _ | _ | 8.2 | 6.5 | 7.6 | _ | 9.4 | 8.4 |
| P | _ | _ | _ | 2.6 | 4.2 | 3.9 | 2.6 | _ | _ | _ | _ | 3.8 | 16 | 2.71 | _ | 5.1 | 5.5 |
| INR | 1 | 1 | 1.19 | 1.06 | 1.23 | 1.1 | 1.1 | 1.3 | 3 | 1.2 | 1 | 1.3 | 1.2 | 1.04 | 1.15 | 1 | 1.08 |
| PT | 12 | 12.5 | 16 | 14.3 | 90 | 13 | 13 | 14.5 | 23 | 13 | 12.9 | 14.5 | 13.5 | 13.9 | 13.5 | 13 | 14.5 |
| PTT | 66 | 65 | 36 | 40 | 44 | 40 | 28 | 79 | 120 | 56 | 31 | 29 | 31 | 36 | 29 | 30 | 25 |
| Mg | _ | 2.3 | _ | 2.1 | 2.4 | 2.1 | 2.2 | _ | 2.1 | _ | _ | _ | 1.2 | 3 | 0.97 | 1 | 2.3 |
| Highest urea | 121 | 106 | 134 | 77 | 73 | 181 | 97 | 145 | 35 | 38 | 80 | _ | 255 | 32 | 78 | 158 | 42 |
| Highest Cr | 9 | 10.9 | 9.61 | 7.69 | 9.1 | 13.8 | 10.6 | 8 | 4.5 | 4 | 11.5 | 6.9 | 17 | 7.3 | 9.7 | 10.14 | 7.2 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ground-glass opacities | + | + | + | + | + | + | + | + | + | + | + | _ | + | + | + | + | + |
| Consolidation | + | + | _ | _ | _ | + | + | _ | _ | _ | + | _ | _ | _ | _ | _ | + |
| Crazy paving | - | + | _ | + | + | + | _ | _ | _ | + | _ | _ | _ | _ | _ | _ | _ |
| Air Bronchogram | + | _ | _ | _ | _ | + | + | _ | _ | _ | _ | _ | _ | _ | _ | _ | + |
| Sub-pleural transparent line | _ | _ | + | _ | _ | _ | _ | + | _ | _ | + | _ | + | + | + | + | _ |
| Halo sing | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Reversed Halo sign | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Nodular opacities | + | _ | _ | _ | _ | + | _ | _ | _ | _ | _ | __ | _ | + | _ | _ | _ |
| Reticulo-nodular involvement | _ | _ | + | + | + | _ | _ | _ | _ | + | _ | _ | _ | _ | _ | _ | _ |
| Tree in bud appearance | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Peribronchovascular involvement | + | _ | _ | _ | + | _ | + | _ | _ | + | + | _ | _ | _ | _ | _ | + |
| Bronchiectasis | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | _ | _ | + |
| Cavitation | + | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Unilateral involvement | _ | + | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | + | _ | _ | _ | _ |
| Unifocal involvement | _ | + | + | _ | _ | _ | _ | + | _ | _ | _ | _ | + | _ | + | _ | _ |
| Peripheral involvement | _ | + | + | + | + | + | _ | + | + | + | + | _ | + | + | + | + | _ |
| Anterior | _ | + | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | _ | _ | _ | _ |
| Bilateral | + | _ | + | + | + | + | + | + | _ | + | + | _ | _ | + | + | + | + |
| Multifocal | + | _ | _ | + | _ | + | + | _ | + | _ | + | _ | _ | + | _ | + | _ |
| Central | + | _ | _ | _ | _ | _ | + | _ | _ | + | _ | _ | _ | + | _ | _ | + |
| Diffuse | _ | _ | _ | _ | _ | _ | _ | _ | _ | + | _ | _ | _ | _ | _ | _ | _ |
| Posterior | _ | _ | + | + | + | + | + | + | + | + | + | _ | + | + | + | + | + |
| RUL | + | + | _ | ++ | _ | + | + | _ | + | +++ | + | _ | _ | _ | _ | ++ | ++ |
| RML | + | +++ | _ | + | _ | + | + | _ | + | +++ | ++ | _ | + | + | _ | ++ | ++ |
| RLL | +++ | + | ++ | + | ++ | ++ | ++ | + | + | +++ | +++ | _ | + | ++ | + | ++ | ++ |
| LUL | + | _ | _ | _ | _ | +++ | +++ | _ | _ | +++ | + | _ | _ | + | _ | ++ | ++ |
| LLL | + | _ | ++ | _ | ++ | ++ | ++++ | + | _ | +++ | +++ | _ | _ | ++ | + | ++ | ++ |
| Pleural effusion | _ | _ | _ | _ | _ | _ | + | _ | _ | + | _ | _ | _ | _ | _ | _ | _ |
| Mediastinal lymphadenopathy | + | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Number of involved lobes | 5 | 3 | 3 | 3 | 2 | 5 | 5 | 3 | 3 | 5 | 5 | 0 | 2 | 4 | 2 | 5 | 5 |
| Lung score | 7 | 5 | 4 | 4 | 4 | 9 | 11 | 2 | 3 | 15 | 10 | 0 | 2 | 6 | 2 | 10 | 10 |
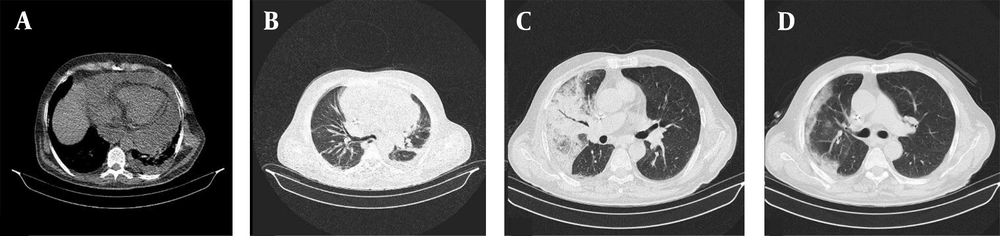
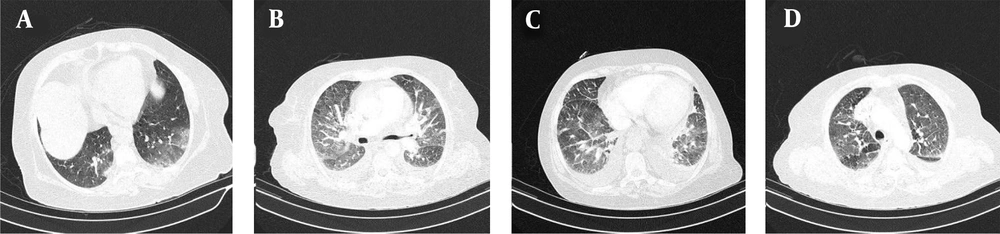
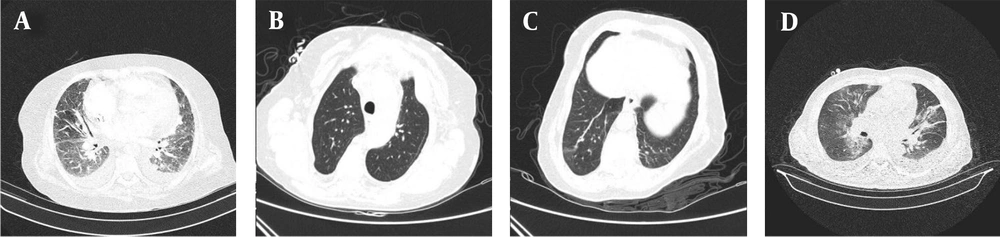
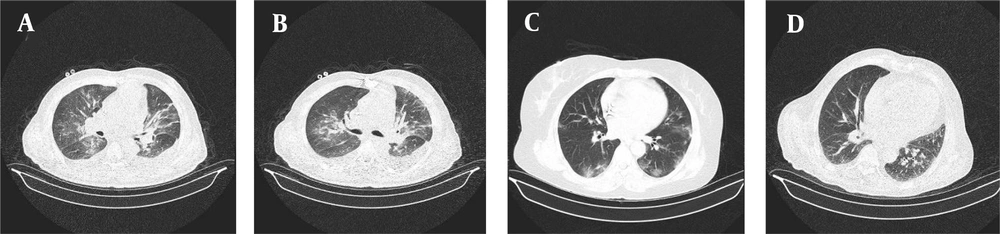
All patients received a single treatment protocol consisting of supportive oxygen via nasal routes or by a mask and received the standard medication before being infected with the virus. All patients received hydroxychloroquine and azithromycin for five days. A single dose of ceftriaxone was also administered to all patients. Three patients who had passed away from the disease also had received subcutaneous interferon beta-1b. Patients number 2, 4, 7, 10, 11, and 16 had received a dose of corticosteroids. Figures 1-4 demonstrate some imaging findings in the patients. Figure 5 shows the relationship between the number of lung lobes involved and the lung score.
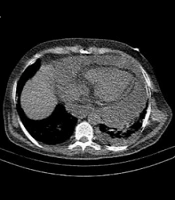
A, Cardiomegaly with pleural and pericardial effusion and sub-segmental atelectasis. B, Subsegmental atelectasis and subpleural band sign with peribronchovascular thickening. C, Right middle lobe airspace consolidation with evident air bronchogram. D, Subpleural ground-glass opacities with interlobular septal thickening causing crazy paving appearance. Pulmonary artery dilatation is also seen.
4. Discussion
The present study assessed the clinical and imaging findings of ESRD patients undergoing hemodialysis who were infected with COVID-19. We showed that the clinical signs and symptoms resembled those of general adult patients hospitalized because of COVID-19. Besides, although the general involvement pattern resembled that of normal adults, some specific imaging signs were witnessed more frequently in this group. Of 17 patients, three died because of the virus, which is higher than the rate expected for the general public. In a hospital study in New York City on 5,449 COVID-19 patients, 36.6% developed AKI, of whom 14.3% required hemodialysis (or other forms of dialysis), and 96.8% of these patients underwent mechanical ventilation. Risk factors for AKI included older age, diabetes, cardiovascular disease, hypertension, need for mechanical ventilation, and vasopressor support (13).
Further evaluations, such as CT imaging during hospitalization and cultures, revealed superinfection with MRSA species. The patients then received prompt antibiotic treatment (vancomycin or teicoplanin plus clindamycin), and eventually, the laboratory and clinical indicators improved in all three patients. However, patient No. 8 experienced acute deterioration and hemodynamic instability in the absence of pneumothorax and arrhythmias and died of suspected massive pulmonary emboli while being on MV. One patient with elevated LFTs was a known case of chronic hepatitis B. Severe ARDS, and pulmonary thromboembolism were the most probable causes of death. The mortality of our patients was not as high as the rate in other case series and studies. We attributed this finding to the admission of all study patients and the common use of corticosteroids as adjuvant treatment. Also, using enoxaparin with the concomitant administration of heparin during dialysis sessions may have led to fewer PTE incidents. Also, patients in our study mainly were middle-aged individuals, in contrast to other studies.
A similar study was performed on 11 patients undergoing peritoneal dialysis in the United States. Of all the patients, two passed away, whit a mortality rate similar to ours. Furthermore, of 11 patients, three needed mechanical ventilation and vasopressor administration. The routine treatment administered to patients included hydroxychloroquine and azithromycin, coupled with agents such as tocilizumab, anakinra, and methylprednisolone. More similarities existed between our subjects and the subjects of this study, as bilateral involvement of the lungs on CT imaging was the dominant involvement pattern. Like our study, only a single patient had a clear lung field. The authors did not further discuss the imaging findings (14). Another study on ESRD patients in Turkey included 25 subjects undergoing chronic dialysis. Fifteen patients were females, and 10 were males. Similar to our study, hypertension was the most common pre-existing condition and the most common imaging findings consisted of bilateral lesions, and only a minority had unilateral or singular lesions. Four patients had leukocytosis, and 18 patients had lymphopenia. Similar to our study, all patients reported elevated CRP. Of all patients, five died (20%), which is only slightly higher than that in our study (17%).
A multicenter Italian study on 94 ESRD patients with COVID-19 reported severe disease at the onset of symptoms, fever, cough, dyspnea, and higher CRP levels (> 50 mg/L) as the predictors of death while fever, dyspnea, age of > 7 0, and history of ischemic heart disease were attributable to ARDS development. Overall, 50% of the patients deteriorated to ARDS (15). A Spanish study on 36 patients concluded that the significant differences between the non-survivors and survivors at baseline were higher oxygen saturation, shorter dialysis vintage, and more common use of online hemodiafiltration in the latter group. The non-survivors had progressively rising LDH and CRP levels and worsening imaging disturbances. In this study, there was no statistically significant difference in CT findings at baseline; both groups had bilateral peripheral GGO or unilateral opacities, but during hospitalization, the worsening of the imaging findings happened in 50% of the patients with the worst pulmonary involvement on the seventh day of symptom onset. Unlike the general population, an elevated level of D-dimer was not a predictor of death in these patients (16). In a similar study on five patients, none of the patients died at the end of the study, and all had lymphocytopenia and ground-glass opacities in chest CTs. Interestingly, 80% of these patients had diarrhea, which is considered a much less common symptom among other patients (17). Based on observations from a study from Wuhan on 32 ESRD patients, the most common presentation was a cough, followed by fever (in less than half of the patients), which was even less common among diabetic patients (18). Also, there was more than a 16% mortality rate among MHD patients in another study, but all of the non-survivors died of extra-pulmonary reasons (19). In a Parisian study on 44 patients, almost all had hypertension, and half had diabetes. The overall mortality rate was 27%. In this study, the prognosis was significantly poorer in ICU patients. Other risk factors for death were cough, thrombocytopenia, elevated LDH, and CRP levels (20). Noteworthy, the hematologic parameters of individuals with ESRD should be interpreted because these individuals suffer from multiple pre-existing conditions, which can alter the initial state of the tests (21).
In general, possibly due to the dysfunction of the immune system, the initial presentation is milder in MHD patients than in the general population. However, they are also complicated, with many pre-existing conditions that may make them susceptible to severe disease (16). On the other hand, some pre-existing symptoms in MHD patients are mutual with those of COVID-19 infection, such as fatigue and dyspnea. This overlap could lead to under-diagnosis in this vulnerable population. Thus, repeated screening is recommended for these patients (22).
4.1. Conclusions
Based on data from our patients, it seems that patients under hemodialysis are prone to severe complications of COVID-19, possibly leading to an increased mortality rate in these patients. Although the general involvement pattern is the same, these patients may also present with uncommon CT findings. Laboratory findings of this group closely resemble the general public, with a difference that a more significant proportion of patients have abnormalities.