1. Background
Statistics suggest that more than half of patients with end-stage renal disease are aged 16-45 years, which is the most active stage of life (1). Male and female patients with chronic renal failure who undergo treatments such as hemodialysis often experience sexual dysfunction and multiple other sexual problems (2). Sexual function is a neglected factor despite its undeniable impact on the quality of life (3). In particular, dialysis patients experience sexual dysfunction due to the nature of their disease and its complications (4). Sexual quality of life is essential to sexual health and refers to sexual attraction, interest, participation in sexual activity, and the perception of sexual function in an individual. This concept is intertwined with the level of satisfaction and general quality of life, such that a low sexual quality of life could properly determine the overall health status and quality of life of a community. Among the influential factors in sexual function are age, cultural and religious background, disease history, surgery, pregnancy, lifestyle, childhood events, previous sexual experiences, stress-related emotional problems, physical health, history of sexual trauma/deterioration, and economic status (5).
Dialysis is the last treatment option for kidney failure. Given the rising trend of the patient population undergoing dialysis to increase survival, the associated sexual dysfunction with this treatment method has increased significantly. Since most organic changes are irreversible and may complicate treatment, it is suggested that the lifestyle of these patients be properly modified and a primary assessment of their sexual function be carried out.
The main factors involved in the pathogenesis of impotence in uremic patients include hormonal imbalance, vascular/neurogenic disorders, medication, and psychological problems (6). Men and women with kidney failure are commonly diagnosed with various sexual and reproductive disorders, such as erectile dysfunction in men, decreased libido, and female infertility caused by ovulation disorders (7, 8). The chemical changes occurring in the body due to renal failure largely affect hormones, nerve function, and the energy level, while they are also associated with impotence and decreased libido. Moreover, physical changes may adversely affect the perception of sexual attraction in these patients. The severity of the disorder could range from a slightly decreased libido to failure to reach orgasm (9). Therefore, it seems that the quality of life of dialysis patients and their sexual function are significantly lower compared to the normal population, especially in men (3).
Impotence is extremely prevalent among dialysis patients, and 33% of patients with renal failure are reported to have no marital relationships. In a research in this regard, 84% and 59% of female and male dialysis patients were diagnosed with sexual dysfunction (10). This issue has also been reported in 80% of hemodialysis patients in the developing countries, with 11% considered to be severe (4). In addition, sexual dysfunction has an extremely high prevalence in female hemodialysis patients and is associated with aging, dyslipidemia, and depression. The quality of life of hemodialysis women is physically lower compared to non-dialysis women (2).
Notably, a lack of interest in sexual relationships in uremic patients is not surprising due to their chronic fatigue, anxiety, and low self-confidence. Evidence attests to a reverse correlation between post-dialysis blood urea nitrogen (BUN) levels, orgasmic function, and sexual satisfaction (6). In other words, hemodialysis patients suffer from chronic fatigue, anxiety, and low self-esteem, which may lead to the reduction of libido and sexual intercourse frequency (11). While hemodialysis is a relatively effective treatment for these patients, in these patients it rises unfavorable complications, such as uremic pruritus, sleep disorders, hypotension, and poor quality of life. Pruritus is defined as an unpleasant sensation that provokes the desire to scratch and is often an irritating, constant symptom in patients with renal failure (60 - 80%). Moreover, it has an adverse impact on the sleep patterns, mood, and quality of life of these patients.
Cold hemodialysis could relieve uremic pruritus in hemodialysis patients (12). The majority of the patients with renal failure who undergo long-term hemodialysis have complaints of sleep disorders, which may be associated with dire complications and low quality of life in these patients, thereby leading to depression, anxiety, and mortality (13). Cold dialysis could improve the sleep quality of patients undergoing hemodialysis (14).
Restless legs syndrome (RLS) is one of the causes of sleep disorders that frequently occurs in dialysis patients, reducing their quality of life (15). Cold dialysis could be used as a non-pharmacological method to reduce the severity of RLS in hemodialysis patients (16). According to the literature, physical, psychological, and social disorders may cause sexual dysfunction, which is highly common among dialysis patients (9, 17). Lowering the dialysate temperature to 35°C from the standard temperature of 37°C leads to the stabilization of hemodynamic variables in dialysis patients without reducing the hemodialysis effects (18).
Cold hemodialysis results in hemodynamic stability during hemodialysis, decreased uremic pruritus, and reduced sleep disorders and RLS, all of which are associated with daily fatigue. Given the association of sexual dysfunction and variables of fatigue and BUN, sexual dysfunction should be considered a major health concern in hemodialysis patients. It has been hypothesized that the reduction of physical complications by cold dialysis could improve sexual function.
2. Objectives
The present study aimed to evaluate the effect of cold dialysis solutions on the sexual dysfunction of hemodialysis patients.
3. Methods
3.1. Design
This triple-blind, two-group, randomized clinical trial was conducted with a before and after parallel design among patients undergoing hemodialysis at Nikan Farda and Tabarsi dialysis centers in Mashhad, Iran, during May 22-September 22, 2020 (Iranian Registry of Clinical Trials code: IRCT20200124046235N1).
3.2. Participants
The sample size was estimated at 30 subjects per group based on the research by Balaban et al. (19), the minimum sample size required for a study at 95% confidence interval, and 85% test power using G*Power statistical software. The participants were allocated to the experimental (dialysis at 35.5°C) and control (dialysis at 37°C) groups using the random block method.
3.3. Procedure
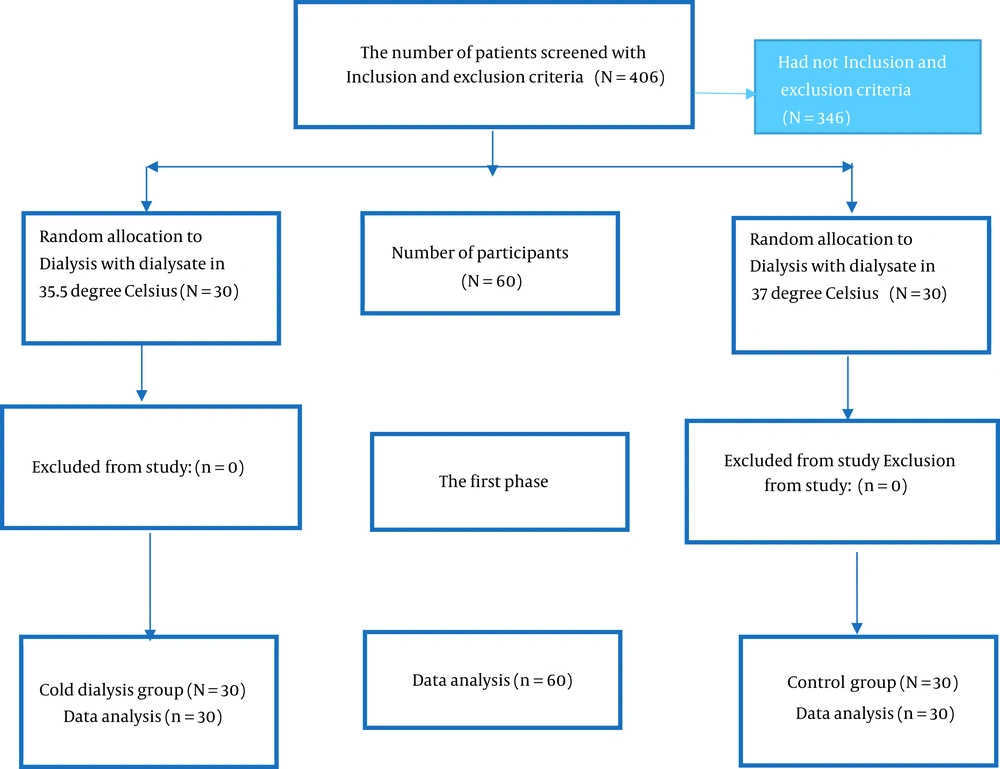
The inclusion criteria of the study were being married, having a sexual partner, and undergoing hemodialysis at least twice per week. The exclusion criteria were as follows: (1) using drugs that affect sexual function, (2) surgery of the genital area due to sexual dysfunction, (3) lack of consent, (4) immigration, (5) hyperparathyroidism, (6) Bisexuality, (7) anemia (hemoglobin < 9), and (8) severe depression. In addition, the exclusion criteria during the intervention were as follows: (1) kidney transplant, (2) death of the patient, (3) death of the spouse/sexual partner, (4) divorce, and (5) unwillingness to cooperate with the researcher (Figure 1).
3.4. Data Processing
Data were collected using the female sexual function index (FSFI), the International Index of Erectile Functions, and a demographic questionnaire. The FSFI is a 19-item tool, and the reliability and validity of its original version have been confirmed (20). The instrument evaluates female sexual function in the six domains of sexual desire (two items), arousal (four items), lubrication (four items), orgasm (three items), satisfaction (three items), and pain (three items). The sexual desire items are scored within the range of 1 - 5, and the items in the other domains are scored within the range of 0-5. The total score of each domain is calculated based on its coefficient, which has been previously determined for sexual desire (0.6), arousal (0.3), lubrication (0.3), orgasm (0.3), satisfaction (0.4), and pain (0.4). The score range of the sexual desire domain is 1.2-6, and the score range of the other domains is 0-6. Moreover, the total score range of sexual dysfunction is 1.2 - 36. In the FSFI, higher scores are indicative of better sexual function. The cutoff points for the domains of sexual desire, arousal, satisfaction, orgasm, lubrication, pain, and overall sexual function have been estimated at 2.1, 2.8, 2.6, 2.8, 2.8, 1, and 28, respectively.
The international index of erectile functions (IIEF) is applied to evaluate male sexual function. With 15 items in five areas, the instrument assesses erectile function, orgasm function, sexual desire, sexual satisfaction, and overall sexual satisfaction. In this tool, higher scores are indicative of better sexual function. The validity of the IIEF has been confirmed by Gorsane at 0.86 (21). The items of the tool are scored based on a five-point Likert scale, and the maximum acceptable score of 75 shows the optimal sexual status in different areas.
In our study, the severity of sexual dysfunction was determined within the score ranges of 0 - 10 (severe), 11 - 16 (moderate), 17 - 21 (mild-to-moderate), 22 - 25 (mild), and 26 - 30 (no disorder). The reliability of the two instruments was assessed using internal consistency among the items in each area of male and female sexual function and overall function (0.89 > α > 0.85). Since Iranians are mostly reluctant to discuss their sexuality or may deliberately give false answers, the research objectives were fully explained to the participants before the intervention. Afterwards, the sexual function of the participants was recorded based on the results of the demographic questionnaire, IIEF, and FSFI.
The research tools were completed again by the patients after hemodialysis with a cold solution (35.5°C) and a standard solution (37°C) in the experimental and control groups for one month, respectively. Notably, data were collected in a self-report manner, and the patients with visual problems orally answered the questions. The blinding of the researcher was attained by recruiting a co-researcher for data collection. The researcher was unaware of the intervention type and random allocation of the subjects to the experimental and control groups. Furthermore, the subjects were blinded to their assigned groups, which was possible by covering the temperature number of the device shown on display using a two-centimeter piece of paper. The statistical analyst of the research was also blinded to the process by assigning A and B codes to the groups. The analyst was also unaware of the allocation of the patients to the experimental and control groups.
3.5. Statistical Analyses
After coding the forms, data analysis was performed in SPSS version 25 using the Shapiro-Wilk test to evaluate the normal distribution of the data and student t-test for inferential data analysis. In all the statistical analyses, a P-value of less than 0.05 was considered significant.
3.6. Ethics
The study protocol was approved by the Ethics Committee of Sabzevar University of Medical Sciences (code: IR.MEDSAB.REC.1398.086). The patients were assured of confidentiality terms regarding their personal information, and written informed consent was obtained from the subjects prior to enrollment. Participation in the study was voluntary, and no obligation was imposed by the attending physician, nursing staff, and researcher/co-researcher.
3.7. Patient and Public Involvement
Patients and members of the public were not directly involved in the design of this study.
4. Results
Table 1 shows the demographic characteristics and medical information of the patients. Comparison of the experimental and control groups in terms of age, duration of hemodialysis, pre-intervention dialysis adequacy, hemoglobin level, gender, education level, occupation status, history of kidney transplant, diabetes, hypertension, pruritus, sleep disorders, and RLS indicated the homogeneity of the groups (Table 1).
| Statistical Index | Control, N = 30 | Intervention, N = 30 | Statistical Results | ||
|---|---|---|---|---|---|
| Test Result | Degrees of Freedom | P-Value | |||
| Age (y) | 42.57 ± 9.98 | 37.13 ± 9.37 | t = 2.17 b | 58 | 0.03 |
| Duration of hemodialysis treatment (mo) | 43.30 ± 47.45 | 23.77 ± 17.69 | Z = -2.04 c | 0.41 | |
| Adequacy of dialysis (KT/V) before the intervention | 1.20 ± 0.18 | 1.28 ± 0.27 | Z = -2.47 c | 0.11 | |
| Hemoglobin level (mg/dL) | 11.45 ± 1.41 | 11.48 ± 1.80 | t = 0.64 b | 58 | 0.95 |
| Gender | -2.58 d | 1 | 0.11 | ||
| Female | 8 ± 27.70 | 14 ± 46.70 | |||
| Male | 22 ± 73.30 | 16 ± 53.30 | |||
| Education level | Fisher's Exact Test | 0.06 | |||
| Illiterate | 0 ± 0.00 | 1 ± 3.30 | |||
| Basic literacy | 6 ± 20.00 | 1 ± 3.30 | |||
| Secondary | 7 ± 23.3 | 15 ± 50.00 | |||
| Bachelor | 3 ± 10.00 | 9 ± 30.00 | |||
| Master of science and higher | 0 ± 0.00 | 1 ± 3.3 | |||
| Occupation status | Fisher's exact test | 0.09 | |||
| Employed | 2 ± 6.70 | 6 ± 20.00 | |||
| Freelancer | 9 ± 30.00 | 8 ± 26.70 | |||
| Worker | 1 ± 3.30 | 2 ± 6.70 | |||
| Housekeeping | 8 ± 26.70 | 11 ± 36.70 | |||
| Unemployed | 6 ± 20.00 | 0 ± 0.00 | |||
| Retired | 4 ± 13.3 | 3 ± 10.00 | |||
| Kidney transplantation history | 0.88 d | 0.35 | |||
| Yes | 8 ± 26.70 | 5 ± 16.70 | |||
| No | 22 ± 73.30 | 23 ± 76.70 | |||
| History of diabetes mellitus | -1.92 d | 1 | 0.16 | ||
| Yes | 3 ± 10.00 | 7 ± 23.30 | |||
| No | 27 ± 90.00 | 23 ± 76.70 | |||
| History of hypertension | -1.09 d | 0.29 | |||
| Yes | 15 ± 50.00 | 19 ± 63.30 | |||
| No | 15 ± 50.00 | 11 ± 36.70 | |||
| Uremic pruritus | 2.51 d | 0.28 | |||
| Yes | 9 ± 30.00 | 13 ± 43.00 | |||
| No | 19 ± 63.00 | 13 ± 43.00 | |||
| Sometimes | 2± 6.70 | 4 ± 13.30 | |||
| Sleep disorder | |||||
| Yes | 6 ± 20.00 | 8 ± 26.70 | 0.48 d | 0.78 | |
| No | 13 ± 43.30 | 13 ± 43.30 | |||
| Sometimes | 11 ± 36.70 | 9 ± 30.00 | |||
| Restless legs syndrome | -0.95 d | 0.09 | |||
| Yes | 6 ± 20.00 | 7 ± 23.30 | |||
| No | 23 ± 76.70 | 22 ± 73.30 | |||
| Sometimes | 1 ± 3.30 | 1 ± 3.30 | |||
aValues are expressed as mean ± SD.
bt-test
c Mann-Whitney U
d Chi-square
Before the intervention, the mean total score of erectile function was 47.57 ± 4.45 and 49.60 ± 4.16 in the experimental and control groups, respectively. In this regard, the results of independent t-test indicated no significant difference between the study groups (t = 2.124; P = 0.14). One month after the intervention, the mean score of this variable was estimated at 53.92 ± 4.59 and 50.90 ± 6.16 in the control and experimental groups, respectively, demonstrating no significant difference between the groups in this regard (t = 0.035; P = 0.97). In addition, no significant difference was observed between the male subjects of the two groups regarding the domains of erectile function, orgasmic function, libido, sexual satisfaction, and overall satisfaction before and after the intervention (Table 2).
| The Field of Male Sexual Dysfunction | Control | Intervention | t-Test Results (Intergroup Comparison) | ||
|---|---|---|---|---|---|
| t | df | P-Value | |||
| Men's erectile function | 0.034 | 10 | 0.97 | ||
| Before the intervention | 19.06 ± 8.370 | 20.27 ± 5.93 | |||
| One month after the intervention | 20.12 ± 8.89 | 20.36 ± 8.05 | |||
| Paired t-test result (Intragroup comparison) | t = -0.034, df = 10, P-value = 0.97 | t = -2.11, df = 15, P-value = 0.052 | |||
| Orgasmic function | 0.47 | 11 | 0.64 | ||
| Before the intervention | 6.22 ± 3.99 | 7.83 ± 2.918 | |||
| One month after the intervention | 6.94 ± 3.66 | 7.16 ± 3.639 | |||
| Wilcoxon test result (Intragroup comparison) | Z = -0.41, P-value = 0.68 | Z = -0.60, P-value = 0.55 | |||
| Sexual desire | 1.81 | 11 | 0.25 | ||
| Before the intervention | 6.00 ± 2.19 | 6.58 ± 1.97 | |||
| One month after the intervention | 6.55 ± 1.85 | 7.16 ± 3.63 | |||
| Wilcoxon test result (Intragroup comparison) | Z = -0.21, P-value = 0.83 | Z = -1.23, P-value = 0.21 | |||
| Intercourse satisfaction | 0.30 | 11 | 0.77 | ||
| Before the intervention | 4.45 ± 47.57 | 10.16 ± 2.40 | |||
| One month after the intervention | 8.57 ± 4.45 | 9.75 ± 4.80 | |||
| Wilcoxon test result (Intragroup comparison) | Z = -0.40, P-value = 0.78 | Z = -0.60, P-value = 0.44 | |||
| Overall erectile function | 1.75 | 13 | 0.10 | ||
| Before the intervention | 47.57 ± 16.68 | 53.60 ± 13.17 | |||
| One month after the intervention | 53.92 ± 17.09 | 50.90 ± 19.49 | |||
| Paired t-test result (Intragroup comparison) | t = 0.41, df = 9, P-value = 0.69 | t = -1.76, df = 13, P-value = -0.10 | |||
aValues are expressed as mean ± SD.
Before the intervention, the mean FSFI score of the control and experimental groups was 17.26 ± 2.31 and 20.65 ± 1.09, respectively. The results of Mann-Whitney U test indicated no significant difference between the groups in this regard (z = 1.12; P = 0.26). One month after the intervention, the mean FSFI score was estimated at 19.11 ± 1.29 and 21.74 ± 1.67 in the control and experimental groups, respectively, showing no significant difference between the groups (z = 1.11; P = 0.27). In addition, no significant difference was observed among the female patients in the domains of desire, lubrication, satisfaction, and pain before and after the intervention. However, the score of the arousal domain in the experimental group changed from 3.68 ± 0.38 before the intervention to 3.98 ± 0.46 after the intervention, exhibiting a significant difference in this regard (z = 2.21; P = 0.027) (Table 3).
| Female Sexual Dysfunction | Control | Intervention | t-test Results (Intergroup Comparison) | ||
|---|---|---|---|---|---|
| t | df | P-Value | |||
| Arousal | Z = -0.541 | 0.58 | |||
| Before the intervention | 2.73 ± 0.69 | 3.68 ± 0.38 | |||
| One month after the intervention | 3.25 ± 0.70 | 3.98 ± 0.46 | |||
| Paired t-test result (Intragroup comparison) | Z = -2.38, P-value = 0.02 | Z = -0.37, P-value = 0.71 | |||
| Desire | Z = -1.13 | 0.25 | |||
| Before the intervention | 2.92 ± 0.52 | 3.30 ± 0.28 | |||
| One month after the intervention | 2.90 ± 0.44 | 4.37 ± 0.32 | |||
| Wilcoxon test result (Intragroup comparison) | Z = -0.66, P-value = 0.51 | Z = -0.84, P-value = 0.40 | |||
| Orgasm | Z = -0.69 | 0.42 | |||
| Before the intervention | 3.05 ± 0.45 | 3.48 ± 0.19 | |||
| One month after the intervention | 3.00 ± 0.39 | 3.40 ± 0.29 | |||
| Wilcoxon test result (Intragroup comparison) | Z = -1.03, P-value = 0.30 | Z = -1.59, P-value = 0.11 | |||
| Lubrication | Z = -0.73 | 0.46 | |||
| Before the intervention | 2.70 ± 0.43 | 2.72 ± 0.13 | |||
| One month after the intervention | 3.30 ± 0.30 | 2.78 ± 0.24 | |||
| Wilcoxon test result (Intragroup comparison) | Z = -0.36, P-value = 0.72 | Z = -0.69, P-value = 0.49 | |||
| Satisfaction | Z = -1.59 | 0.11 | |||
| Before the intervention | 3.25 ± 0.69 | 4.54 ± 0.30 | |||
| One month after the intervention | 4.33 ± 0.61 | 4.68 ± 0.42 | |||
| Paired t-test result (Intragroup comparison) | t = 0.41, df = 9, P-value = 0.69 | t = -1.76, df = 13, P-value = -0.10 | |||
| Pain | Z = -0.67 | 0.55 | |||
| Before the intervention | 2.60 ± 0.56 | 2.91 ± 0.40 | |||
| One month after the intervention | 2.33 ± 0.71 | 2.33 ± 0.70 | |||
| Paired t-test result (Intragroup comparison) | Z = 0.00, P-value = 0.99 | Z = -0.58, P-value = 0.51 | |||
| Overall function | Z = -1.11 | 0.27 | |||
| Before the intervention | 17.26 ± 2.31 | 20.65 ± 1.09 | |||
| One month after the intervention | 19.11 ± 1.29 | 21.74 ± 1.67 | |||
| Paired t-test result (Intragroup comparison) | Z = -1.05, P-value = 0.29 | Z = -0.94, P-value = 0.34 | |||
aValues are expressed as mean ± SD.
5. Discussion
According to the results of the present study, the score of the arousal domain increased in the experimental group after the intervention. Since no studies have assessed the effects of cold dialysis solution on the sexual dysfunction of hemodialysis patients, our findings could confirm the impact of this procedure on other complications that might have affected patients’ sexual dysfunction. In a study conducted by Ghanbarabadi et al., cold solution dialysis was reported to improve the sleep quality of patients in the experimental group (14).
According to Rad et al., cold dialysis is a non-pharmacological method that could reduce RLS in patients undergoing hemodialysis. Therefore, this method could be applied to improve this syndrome in hemodialysis patients (16). In another study by Sajadi et al., cold dialysis was reported to decrease severe fatigue in dialysis patients (22). According to the results obtained by Rad et al., cold hemodialysis could relieve uremic pruritus in patients (12). In a research, Moujerloo et al. reported an increase in the hemodynamic stability and decrease in the symptoms of hypotension, highlighting the need for treatment measures in patients with hypotension during dialysis following the use of cold dialysis (23).
Based on the aforementioned findings, it seems that cold dialysis may reduce sleep quality, RLS, and fatigue and improve blood pressure and dialysis adequacy, thereby enhancing arousal in patients by increasing their quality of life. While the increased mean libido of the patients in the experimental group after our intervention was not considered significant, it may be clinically significant. Moreover, our findings were indicative of an insignificant increase in the mean scores of orgasmic function, lubrication, sexual satisfaction, and overall FSFI in female subjects in the experimental group after the intervention. On the other hand, the mean score of sexual pain in the female subjects of the experimental group decreased after the intervention, which was not considered significant.
In hemodialysis patients, hormonal and brain changes could lead to sexual dysfunction, along with physical weakness, fatigue, and body image disorders (24). Given the lack of improvement in brain and hormonal changes by cold dialysis, this type of treatment cannot enhance all the domains of physical dysfunction.
5.1. Limitations of the Study
The main limitation of the study was the patients’ shame of attending the research setting, which complicated their convincing and enrollment in the research. Another limitation was the self-report nature of data, since the patients might have exaggerated or not truly expressed their sexual problems. In addition, the duration of the intervention was extremely short, and further longitudinal studies are required in this regard.
5.2. Conclusions
According to the results, cold dialysis solution could only upturn the psychological sexual arousal of the female subjects in the experimental group after the intervention, while it had no impact on the other sexual function domains of the male and female patients. It seems that organic and hormonal disorders may cause complete reproductive system inadequacy in men and women, and it is suggested that further studies be conducted in this regard. Since our research was performed for only one month and the duration would not suffice to demonstrate the physical changes that might have affected the sexual dysfunction of the subjects, it is recommended that further longitudinal studies be carried out on the same subject.