1. Background
Benign prostatic hyperplasia (BPH) is the most common cause of bladder outlet obstruction (BOO) in adult men, especially those older than 50 years. Choosing the right patients for the treatment, especially the surgical approaches, is an important issue. Many existing tests and indicators are used to evaluate the patients, including ultrasonographic evaluation, urodynamic tests, and serum biomarkers, which are most valuable in planning the treatment strategy. However, some of these tests have a number of limitations in clinical practice, especially their relative invasiveness and potentially significant side effects. Thus, among all diagnostic methods, the non-invasive and readily available ultrasound evaluation has been suggested as an effective method (1).
Evaluation of the intravesical prostatic protrusion index (IPP) using ultrasonography indicated the relation between this index and the severity of BOO. In the late 19th century, the first observations on the characteristics of IPP and its association with prostate and urinary tract diseases were reported. However, until the early 21st century, the issue of the close relationship between the IPP and BPH had not yet been raised (1, 2). It was hypothesized that the IPP index, along with other objective and measurable indicators, may be useful for the evaluation and management of BPH. Besides, there was a close similarity between the results of the IPP measurement by the two means of transrectal and transabdominal ultrasonography; therefore, abdominal ultrasonography was suggested as a suitable tool for the determination of IPP. Overall, based on the available data, BOO cannot be accurately assessed, or confirmed only by clinical symptoms, post voiding residual urine (PVR) volume, or maximum urinary flow rate (Qmax) index. In this regard, ultrasonography will be able to accurately evaluate the structural abnormalities of the prostate, especially the BOO, as a widely used and reliable non-invasive method. It seems that the measurement of the IPP index will not only have a high predictive ability to confirm the BOO but also can predict the other clinical and structural abnormalities of the prostate. It seems that the use of IPP indices has high sensitivity and diagnostic accuracy in the clinical evaluation of the prostate, even in comparison to the urodynamic indices (3-5).
2. Objectives
The present study aimed to investigate the clinical significance of the IPP index in BPH patients to clarify its diagnostic value in predicting the clinical and structural abnormalities of the prostate.
3. Methods
This descriptive and analytical cross-sectional study included every man older than 50 years with lower urinary tract symptoms (LUTS), predominantly voiding or obstructive, suggestive of BPH. Patients with a history of neurogenic bladder, diabetes mellitus, lumbar disc herniation, spinal trauma/surgery, urethral stricture/injury, prostate cancer, and those with the absolute indications for surgery were excluded from the study. Informed written consent was taken before the study, based on IR.TUMS.MEDICINE.REC.1396.4107 ethical code of Tehran University of Medical Sciences ethics committee.
After standard clinical approach and making the diagnosis of BPH, the patients were evaluated to determine the following indices: International Prostate Symptom Score (IPSS) index, quality of life (QoL) index obtained by a single question used to determine the "bother score", which provides a widely used and statistically valid measure (6), prostate volume (PV) and PVR assessed by transabdominal ultrasonography, serum PSA level, and the Qmax obtained by uroflowmetry.
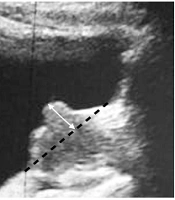
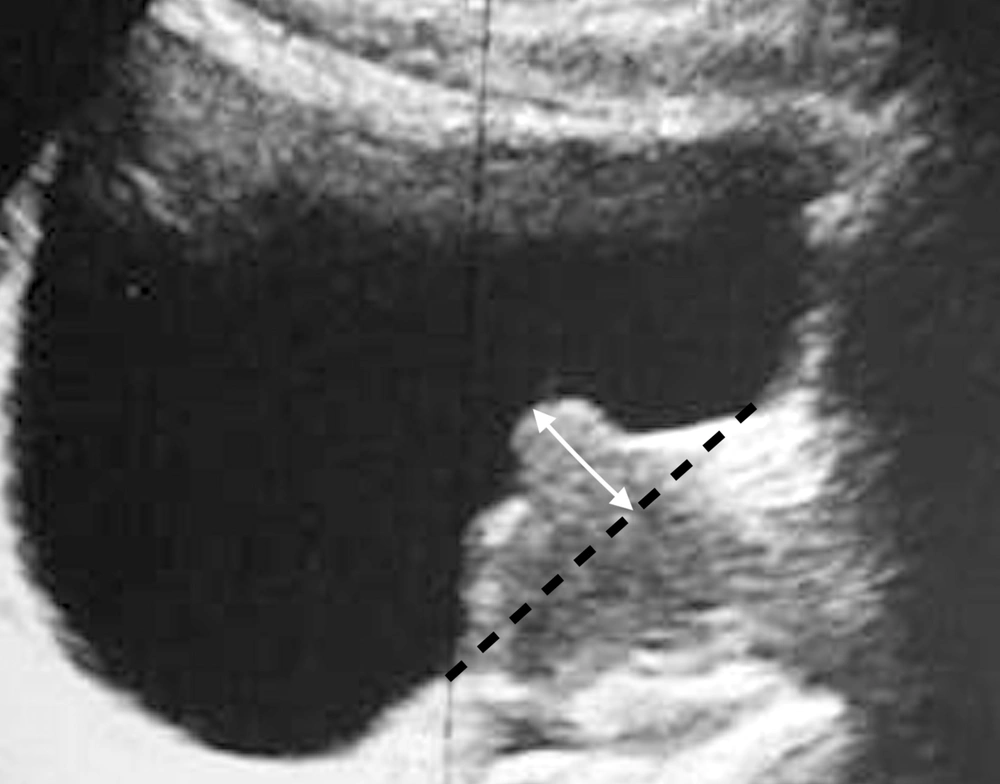
Subsequently, the assessment of the IPP index was undertaken by transabdominal ultrasonography. It should be noted that all ultrasounds were performed by the same physician, and the IPP was defined by the distance between the tip of the prostate's protrusion into the bladder and the bladder neck in sagittal view (Figure 1).
The categorization of the IPP index was done into 3 grades: Grade one (below 5 mm), grade two (between 5 and 10 mm), and grade three (greater than 10 mm).
The results were expressed as mean and standard deviation (mean ± SD) for the quantitative variables and as percentages for the qualitative ones. SPSS software version 23 was used for statistical analysis, and the quantitative variables were analyzed using the t-test or ANOVA test, while the chi-square test was used to compare the qualitative variables.
4. Results
A total of 60 patients with the clinical diagnosis of BPH were monitored in this study; all were treated with tamsulosin 0.4 mg daily. The mean follow-up time was 3.97 ± 1.48 months (ranging from 1.5 to 7 months). Of 60 evaluated patients, 23 (38.3%) received finasteride 5 mg daily, concurrently. During the follow-up period, 10 patients (16.7%) underwent open prostatectomy or transurethral resection of the prostate (TURP).
The mean QoL (bother) scores before the treatment were 1.88 ± 0.88, 2.23 ± 1.09, and 2.95 ± 0.89 for the IPP grades 1, 2, and 3, respectively, indicating a statistically significant difference between the three groups (P = 0.001). Meanwhile, the mean QoL (bother) scores after the medical intervention were 1.76 ± 1.01, 2.08 ± 1.04, and 2.73 ± 1.35, respectively, for grades 1, 2, and 3, which again showed a significant difference (P = 0.020) (Table 1). According to Pearson correlation coefficient, there was a significant direct correlation between IPP and Qol (bother) scores either before (correlation coefficient = 0.459, P < 0.001) or after the intervention (cc = 0.353, P = 0.006). In general, the QoL decreased significantly with increasing the IPP score (Table 2).
| Grade 1 | Grade 2 | Grade 3 | P-Value | |
|---|---|---|---|---|
| Qol | ||||
| Pre-treatment | 1.88 ± 0.88 | 2.23 ± 1.09 | 2.95 ± 0.89 | 0.001 |
| Post-treatment | 1.76 ± 1.01 | 2.08 ± 1.04 | 2.73 ± 1.35 | 0.020 |
| IPSS | ||||
| Pre-treatment | 10.88 ± 5.83 | 12.62 ± 5.04 | 16.36 ± 5.91 | 0.006 |
| Post-treatment | 11.08 ± 4.98 | 11.54 ± 4.14 | 17.27 ± 8.21 | 0.003 |
| PV | ||||
| Pre-treatment | 36.68 ± 16.31 | 34.54 ± 8.64 | 68.18 ± 32.99 | < 0.001 |
| PVR | ||||
| Pre-treatment | 31.20 ± 36.78 | 39.31 ± 40.38 | 69.14 ± 81.05 | 0.038 |
| Post-treatment | 21.68 ± 21.71 | 27.00 ± 26.70 | 61.77 ± 59.89 | 0.004 |
| PSA | ||||
| Pre-treatment | 1.43 ± 0.80 | 1.32 ± 0.68 | 2.79 ± 0.89 | < 0.001 |
| Qmax | ||||
| Pre-treatment | 16.21 ± 4.59 | 12.25 ± 1.50 | 11.17 ± 3.09 | 0.002 |
| Post-treatment | 18.07 ± 5.17 | 13.75 ± 2.99 | 9.83 ± 3.42 | 0.001 |
| Index | Correlation Coefficient | P-Value |
|---|---|---|
| Qol | ||
| Pre-treatment | 0.459 | < 0.001 |
| Post-treatment | 0.353 | 0.006 |
| IPSS | ||
| Pre-treatment | 0.397 | 0.002 |
| Post-treatment | 0.401 | 0.001 |
| PV | ||
| Pre-treatment | 0.504 | 0.001 |
| PVR | ||
| Pre-treatment | 0.293 | 0.029 |
| Post-treatment | 0.399 | 0.002 |
| PSA | ||
| Pre-treatment | 0.566 | < 0.001 |
| Qmax | ||
| Pre-treatment | -0.552 | < 0.001 |
| Post-treatment | -0.695 | < 0.001 |
The mean IPSS scores of patients, before the treatment, for grade 1, 2, and 3 subgroups were 10.88 ± 5.83, 12.62 ± 5.04%, and 16.36 ± 5.91, respectively. In other words, as the IPP increased, the IPSS score also increased (P = 0.006). After providing the medical intervention, the mean IPSS scores were 11.08 ± 4.98, 11.54 ± 4.14, and 17.27 ± 8.21, respectively. (P = 0.003). According to the Pearson correlation coefficient, there was a significant direct correlation between the IPP and IPSS scores, either before (cc = 0.397, P = 0.002) or after the treatment (cc = 0.40, P = 0.001).
Regarding the relationship between the IPP and PV indices (before treatment), mean PV for IPP subgroup grades of 1, 2, and 3 were 36.68 ± 16.31, 34.54 ± 8.64, and 68.18 ± 32.99, respectively. In this regard, the grade 3 patients with IPP > 10 mm had significantly higher PV than the other two subgroups (P < 0.001). There was a significant direct correlation between IPP and PV indices (cc = 0.504, P = 0.001).
In evaluating the correlation between the IPP and PVR indices, the mean PVR of patients before treatment for IPP subgroups of 1, 2, and 3 was 31.20 ± 36.78, 39.31 ± 40.38, and 69.14 ± 81.05, respectively. In other words, as the IPP increased, the patients' PVR also increased (P = 0.038). After the medical intervention, the mean PVR for the IPP subgroups was 21.68 ± 21.71, 27.00 ± 26.70, and 61.77 ± 59.89, respectively; again, indicating a significant difference between the three groups (P = 0.004). According to the Pearson correlation coefficient, there was a significant direct correlation between the IPP and PVR indices before (cc = 0.293, P = 0.029) and after the medical intervention (cc = 0.399, P = 0.002).
The mean PSA level for the IPP grades 1, 2, and 3 was 1.43 ± 0.80, 1.32 ± 0.68, and 2.79 ± 0.89, respectively. Those with an IPP greater than 10 mm had a significantly higher PSA level than the other two subgroups (P < 0.001). In this regard, there was a significant direct correlation between the IPP score and the PSA level (cc = 0.556, P < 0.001).
In evaluating the correlation between IPP value and Qmax index, the mean Qmax of patients before treatment for IPP grade 1, 2, and 3 subgroups was 16.21 ± 4.59, 12.25 ± 1.50, and 11.17 ± 3.09, respectively; which showed that with increasing IPP index, the Qmax of the patients decreased significantly (P = 0.002). Meanwhile, after the medical intervention, the mean Qmax of patients was 18.07 ± 5.17, 13.75 ± 2.99, and 9.83 ± 3.42, respectively, which was again indicating a significant difference between the three groups (P = 0.001). According to the Pearson correlation coefficient, there was a significant inverse correlation between the IPP score and the Qmax both before (cc = - 0.555, P < 0.001) and after the medical intervention (cc = - 0.695, P < 0.001).
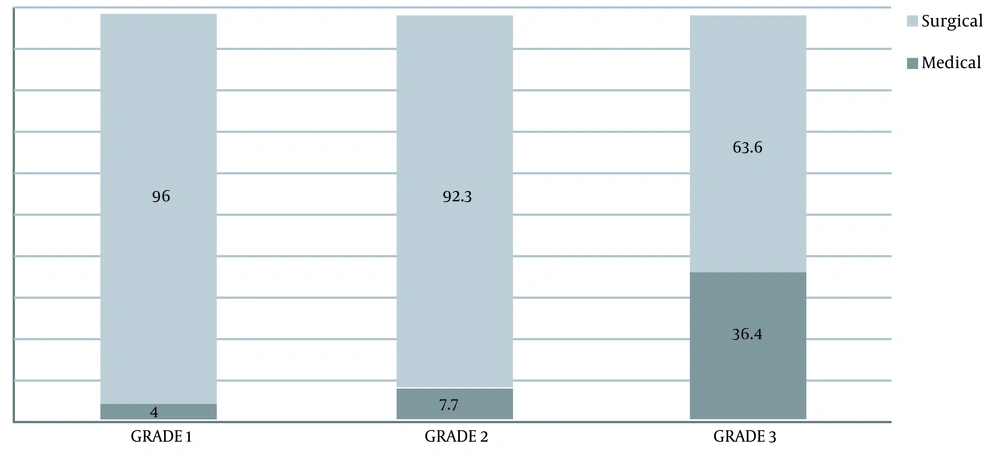
Finally, the prevalence of the need for the surgical intervention in the IPP grades 1, 2, and 3 subgroups was 4%, 7.7%, and 36.4%, respectively, which showed a significant difference between the subgroups; so that the higher the IPP score, the more the need for surgery (P <0.007) (Figure 2).
5. Discussion
Our results show a direct correlation between the IPP index and the QoL, IPSS, PV, PVR, and PSA indices, as well as inversely with the Qmax index before and after BPH medical therapy. Also, according to the findings, the need for surgical intervention increased significantly with the level of IPP.
As mentioned before, there are various invasive and non-invasive indices for evaluating, diagnosing, and predicting the severity of BPH. PSA, PVR, PV, and Qmax are mentioned in this regard. The main reason for the researchers' interest in finding other markers or indicators for BPH is the low sensitivity and specificity of these methods in predicting BPH outcomes and evaluating the severity of BPH-induced BOO, particularly the distinction between BPH and prostate cancer (1). In this regard, special focus has recently been placed on the index of prostate protrusion into the bladder or IPP, and various studies have been conducted on the diagnostic value of IPP in diagnosing and predicting BPH and its severity (7-9).
Whatever emphasized in the studies, particularly in the present study, was the correlation between IPP and other indicators used in the BPH assessment mentioned above. What we did in the present study was to evaluate the correlation between IPP and the classification provided for it with other indices used to assess BPH and predict the need for surgical intervention in these patients. There was no similar study on the need for surgical intervention during the follow-up period. However, the small sample size and the relative shortness of the follow-up period are shortcomings of our study.
As mentioned, similar studies have yielded similar results to our findings. In the study of Liu et al., and contrary to our study, although there were significant changes in total prostate volume and volume of the transitional zone following drug treatment, no significant change was observed in the degree of IPP (10).
In the study of Suzuki et al., which is quite similar to our study, the BOO index was positively correlated with the IPP index. Among all above indices, the highest ability to predict BPH was related to IPP (11).
Wang et al. showed a strong and significant relationship between prostate volume and IPP degree. There was also an inverse relationship between IPP and Qmax, which was consistent with our results (12). In the study by Lee et al., the two indices of total prostate and transitional zone volume were different between the two groups of IPP, which is similar to our study, but the change in other indices such as Qmax and PVR was not correlated with IPP, which is not consistent with our study (13). What seems to be responsible for different findings of various studies is the factors related to the experience of the operator evaluating the ultrasonographic or urodynamic indices, as well as the different sample sizes and power of the mentioned studies.
Recently a systematic review has evaluated the role of IPP in determining BOO and unsuccessful trial without catheter (TWOC) in 4128 patients. The role of IPP in UDS-determined BOO is investigated in 1478 patients, and the role of IPP in predicting unsuccessful TWOC is examined in 2650 patients. Finally, the authors concluded that based on evidence, IPP index (at a cut-off of >10 mm) is strongly associated with BOO and failed TWOC. But in this meta-analysis, the role of IPP in predicting the need for surgery has not been investigated. For the first time, we examined the role of IPP in predicting the need for surgery, and we think this may be a reliable clinical parameter for this purpose. Our results regarding the correlation between IPP and BOO are similar to the results of this systematic review (14).
In this study and line with previous studies, a direct correlation between IPP and QoL, IPSS, PV, PVR, and PSA indices was confirmed, both before and after the treatment for BPH. Also, the inverse correlation between IPP and Qmax before and after the medical intervention was confirmed (15, 16). In other words, it can be concluded that the amount of prostate gland protrusion into the bladder is physically correlated with the severity of the BPH, increasing the size of the prostate, decreasing the QoL of patients, and decreasing the flow of the bladder outflow and, as a representative of each of the above indicators, can be used to evaluate the BPH and its consequences.
5.1. Conclusions
This study demonstrated that the IPP index has a direct correlation with QoL, IPSS, PV, PVR, and PSA indices. Additionally, this parameter had a negative correlation with the Qmax index. These associations were observed both before and after medical treatment of BPH. We also found that the need for surgical intervention increased significantly with increasing IPP grades. Based on this result, we think that the IPP index can be useful for assessing and predicting the severity of symptoms, outcomes, and the need for surgery in patients with clinical BPH. However, studies with larger sample sizes and longer follow-up should be performed for better decision-making.