1. Introduction
Cancer of urethra (UC) is an infrequent entity. It is estimated to constitute less than 1% of all detected cancers (1, 2). The frequency of its occurrence is three times higher in men than in women since the male urethra is much longer than the female. The small number of UC cases makes it difficult to investigate it. The centers have reported small groups of patients at different stages of the disease, which also complicates the comparative analysis. Cancer of urethra arises from different types of cells present in the male urethra depending on the anatomy and location of the tumor in the urethra. A dominant variant is the urothelial type in about 55 - 65% of all cases, which is seen in the proximal part. Less frequent variants are squamous cell tumors (16 - 22% located in the distal segment and adenocarcinomas in 10 - 16% of the cases) (3-5). Cancers from squamous cells are associated with human papilloma virus (HPV) infection (4, 5). The rarest ones are melanoma and sarcoma, found in 1% of the cases (6). The most common symptoms are urethral bleeding, obstructive voiding, and regional pain. Palpable mass in the perineum or penis and inguinal lymphadenopathy are late symptoms (1, 6). Risk factors for developing cancer are chronic inflammation, strictures, previous injury, and HPV infection (4, 5). The treatment method is selected based on the stage, location of the tumor, and malignancy grade of cancer (4, 5). Surgery is the primary and most effective method, but radiotherapy is also performed as the main or additional therapy. Chemotherapy as neoadjuvant or adjuvant therapy can support TCC based on the stage (1, 4, 7).
2. Case Presentation
2.1. Patient 1 (K. W. 68 y)
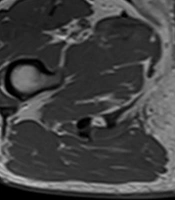
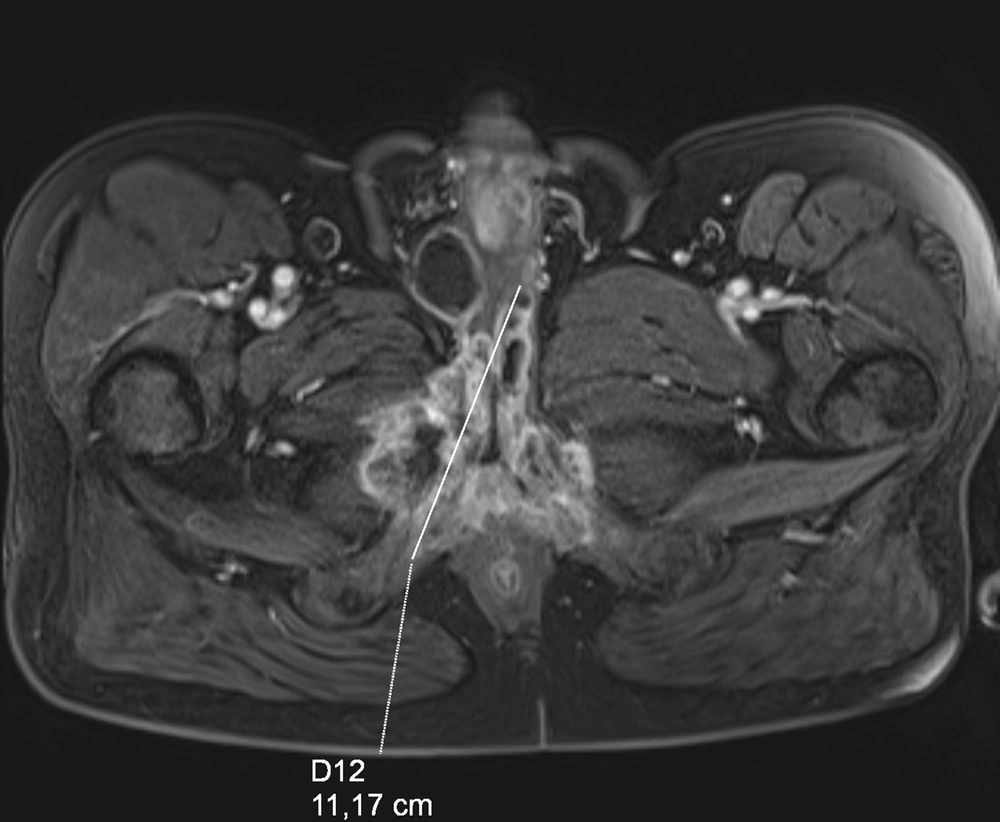
The patient was admitted to the regional hospital in September 2018 due to difficulties in voiding and pain. He had swelling of the lower limbs as well as swelling of the scrotum and haematuria. According to his medical history, he had type 2 diabetes, hypertension, a heart attack, and stenting in 2017, as well as gout and chronic obstructive pulmonary disease. The patient underwent spinal surgery, knee support, and cholecystectomy. He lost weight from 108 kg to 75 kg in two years. In the ultrasound examination TAUS, an unclear picture of the pelvis and bladder neck was snapped, and, therefore, MRI was performed (Figure 1). The pathological tumor in the proximal urethra was discovered infiltrating the spongious body, and no enlarged lymph nodes were observed. Only one node suspected to be 10 mm in the area of the left groin was determined. The biopsy was taken from the urethra during urethroscopy (10.09.2018), but neoplasmatic cells in the material were not confirmed, and only inflammatory and necrotic changes were recorded. The second attempt was made using a transperineal core needle biopsy (17.10.2018). The nests of urothelial carcinoma cells were included in the histopathology report. In January 2019, the patient developed urinary retention, and a cystostomy was created due to the difficulty of inserting a Foley catheter. The patient received two cycles of neoadjuvant chemotherapy with cisplatin and gemcitabine - PG scheme, and the single lymph node decreased from 10 to 6 mm. In March 2019, he was admitted to our urology department, and a penectomy was performed. During surgery, infiltration of the pubic symphysis was discovered. The pathology result showed -pT3 high-grade urothelial carcinoma. Unfortunately, lateral margins of resection were positive, and the patient was qualified for additional radiotherapy. However, he did not start radiotherapy and died in June 2019 due to the progression of cancer.
2.2. Patient 2 (B. J. 67 y)
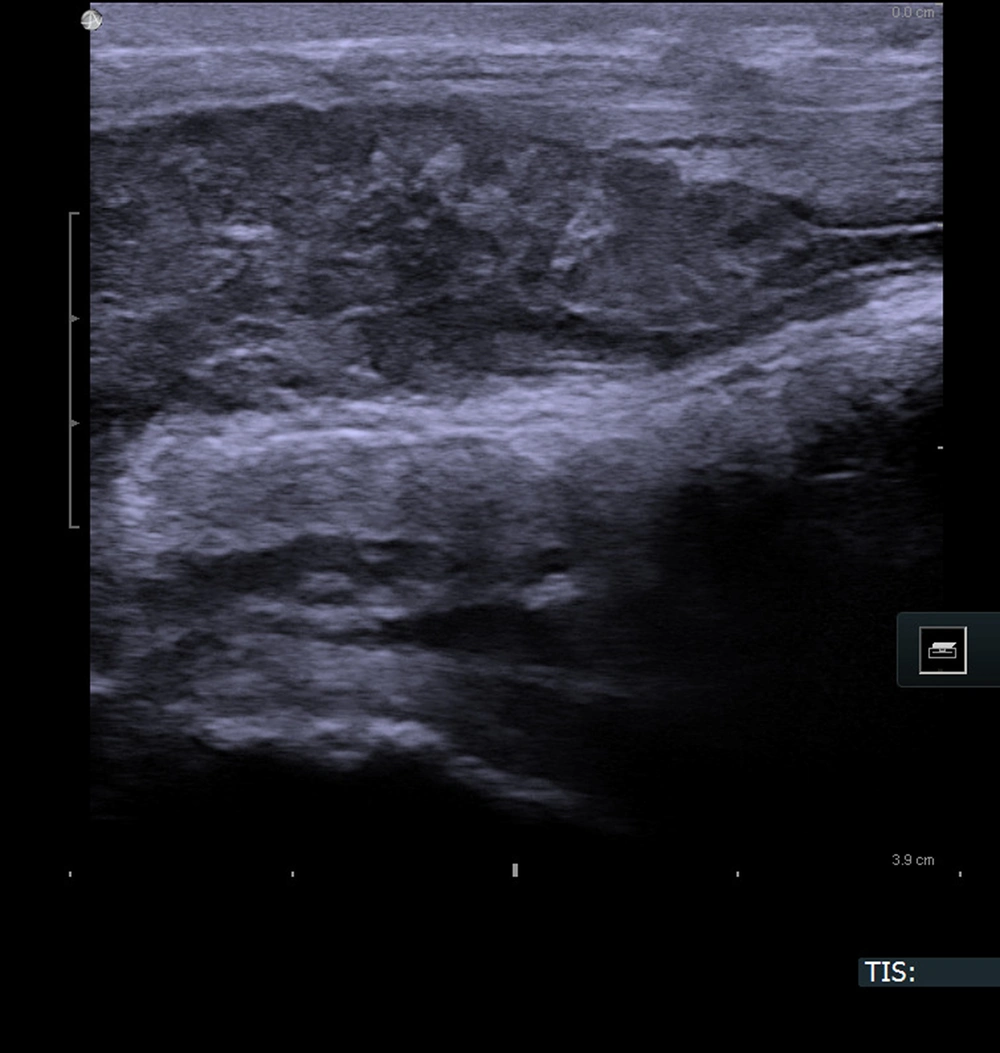
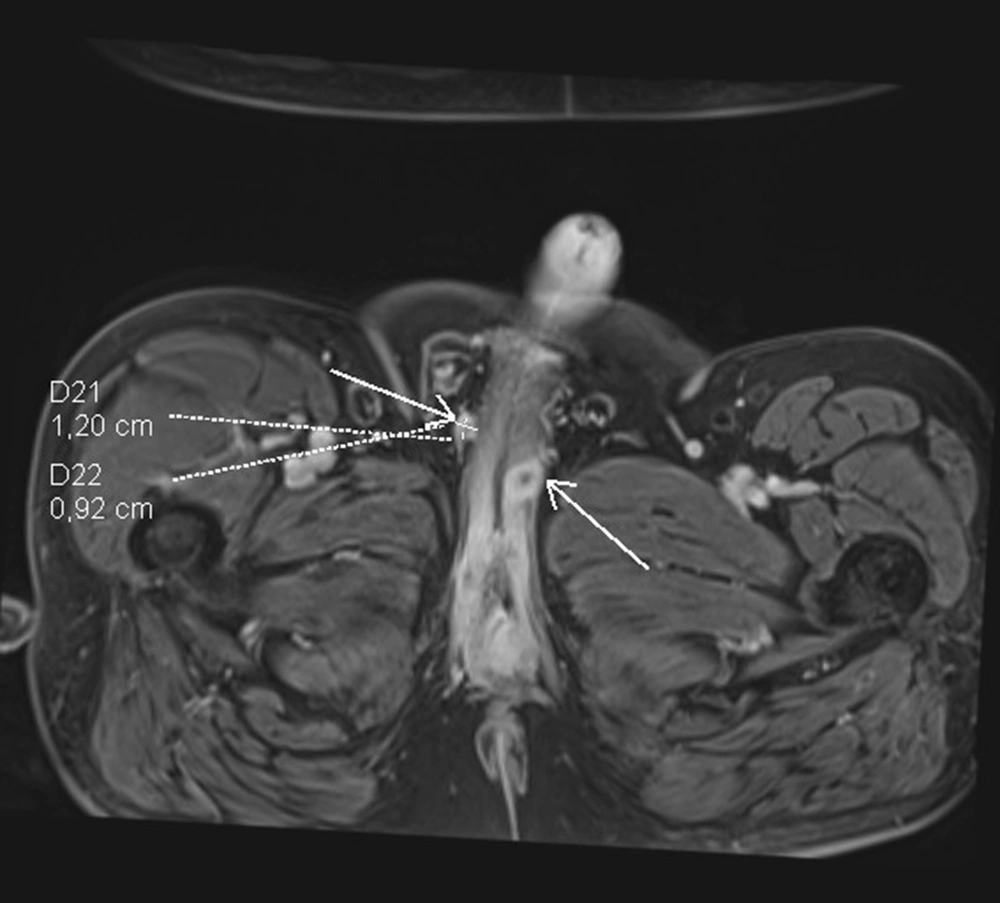
The patient was first admitted to our department in January 2019 due to phimosis. He was not chronically ill and, therefore, was not given medications and was not considered for the operation. According to his medical history, he had an injury to the penis in 2008 by an angle grinder. During the circumcision procedure, a stricture in the distal urethra was discovered. Pathology report described lichen sclerosis. His second hospital stay was in March 2019 due to difficulty in urinating. Ultrasound of the urethra (sonourethrography) was performed, and a 35-mm-long stricture of the distal part and pathological mass from the bulbar urethra to the pelvic diaphragm were found. A colour doppler was performed, and the mass was detected to have pathological circulation. Surgery was performed, stricture was longitudinally slit, and cystoscopy was performed. The closing tumor was present in the proximal urethra, thereby biopsy was conducted adopting TUR and cystostomy. Pathology report revealed squamous cell carcinoma, but the exclusion was not possible since it was urothelial tumor with squamous differentiation. The patient underwent radical radiochemotherapy (05-06.2019) and received RT IMRT Dg 66 Gy/30 Fr as well as five cycles of cisplatin-based CHT 40 mg/m2. Control MRI was carried out in July 2019, and an extensive periurethral infiltration, including penile urethra with diffusion restriction and contrast enhancement features, was determined. The lymph nodes were not enlarged, and no distant metastases were found (Figure 2). The next MRI (February, 2020) showed infiltration 28 × 20 mm surrounding the proximal urethra like a necrotic residual tumor and a small unclear tumor on the glans of the penis. According to another MRI (August, 2020), a fluid reservoir 47 × 19 mm near the bulbar urethra was determined, and a nodule suspected of recurrence was visible on the left side of the penis. The patient's clinical condition deteriorated gradually, and he was re-hospitalized in August 2020 in the Oncology Department due to the disease recurrence. He received a further course of chemotherapy, but it did not stop the disease progression, and he died in December 2020.
2.3. Patient 3 (N. M. 55 y)
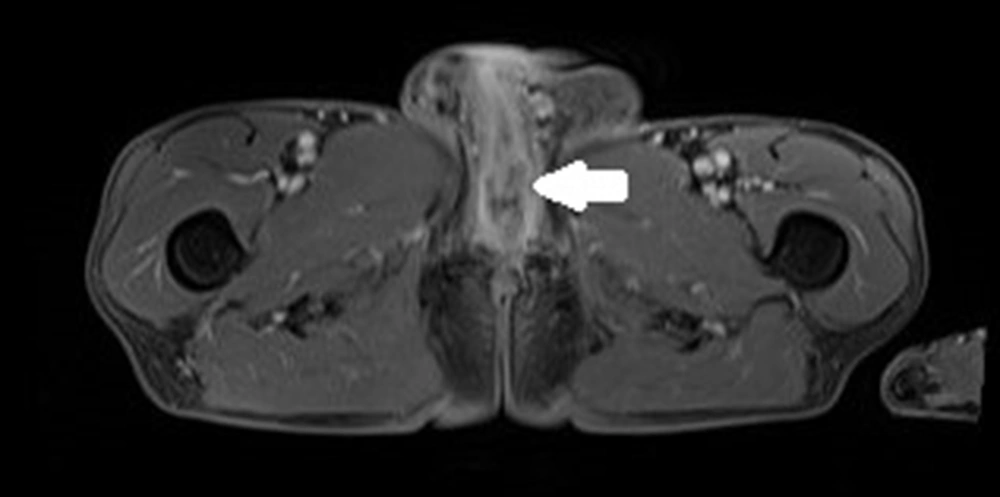
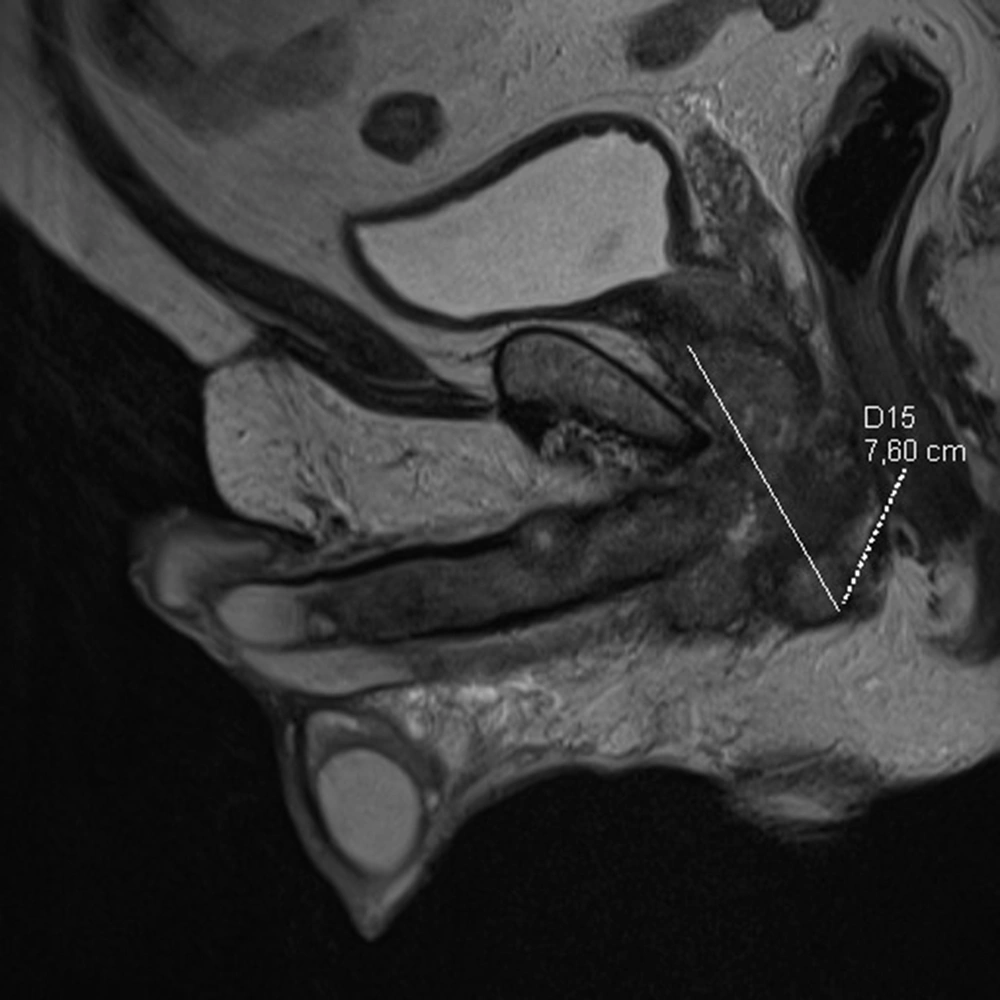
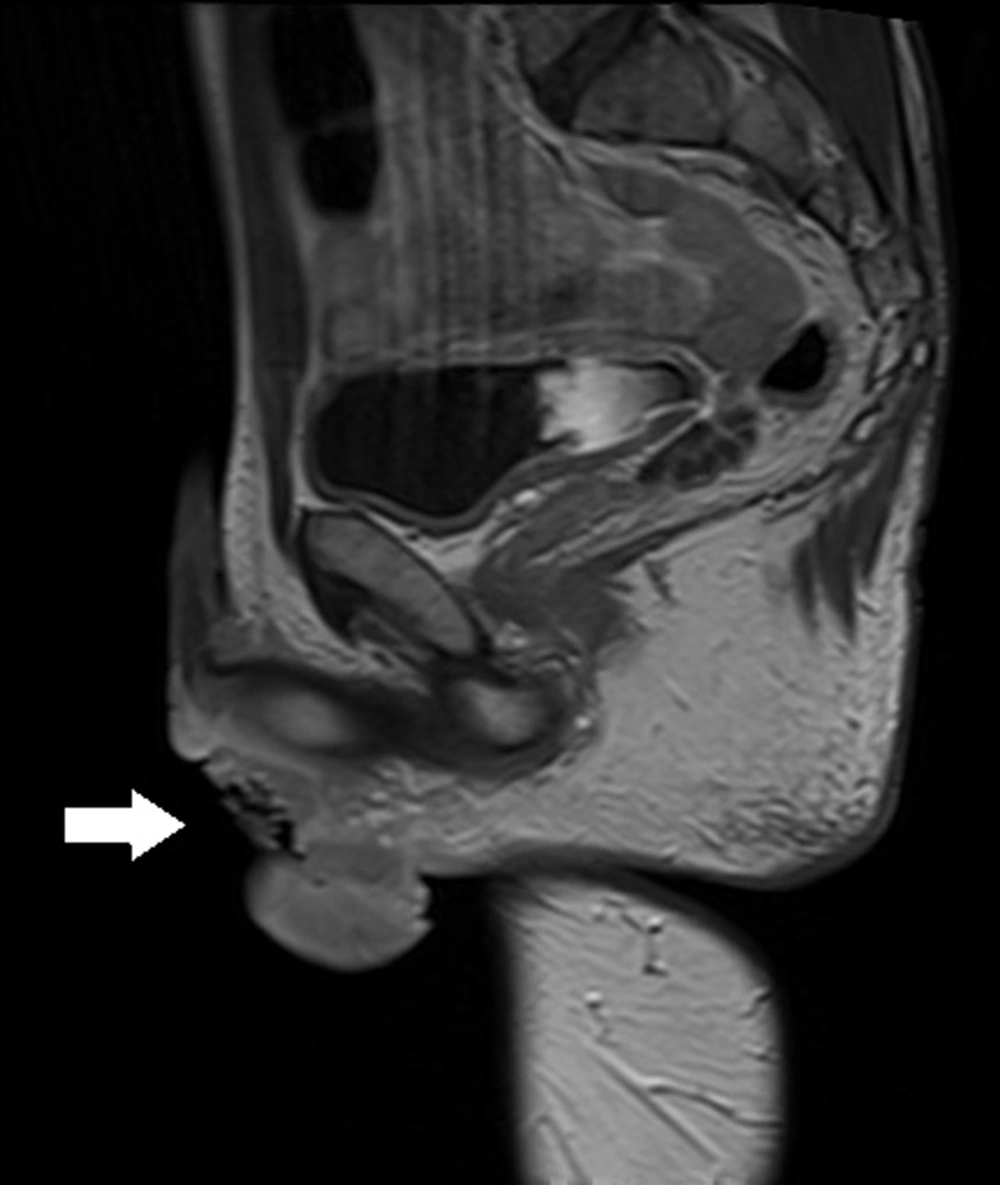
The patient was first admitted in April 2020 due to haematuria reported in February 2020 and due to a palpable mass at the base of the penis. Based on his medical history, he was already treated for arterial hypertension with ramipril 10 mg and hypothyroidism with levothyroxine 50 microgram for several years due to obstructed micturition and was also given tamsulosin and finasteride. The medical history also showed that the patient underwent an excision of urethral stricture in 2014 and had hydrocele surgery in 2015. In May 2019, he underwent an E coli infection and was treated with levofloxacin for 30 days. A while later, the patient reported severe erectile dysfunction, and his MRI showed the polycyclic tumor 76 × 67 × 31 mm with post-contrast enhancement (Figure 3). The tumor covered the bulbar urethra, invaded the urethra and corporal crura and probably muscle levator ani, and adhered to the prostate. Lymph nodes were enlarged up to 18 × 12 mm in both inguinal areas. Uroflowmetry was performed, and Q max was decreased to 7.7 mL/sec. Escherichia coli 10/6 was present in the urine culture. Urethroscopy and transurethral biopsy from the tumor were performed, and a cystostomy tube was inserted into the bladder. No cancer cells were found in the pathology examination, and only active inflammatory infiltration was determined. The patient was discharged from the hospital and treated with an antibiotic for further treatment on an outpatient basis. In May 2020, the urethrocystography was carried out, and extravasation of contrast in the bulbar urethra was discovered, thereby, he was recommended to urinate only by cystostomy and undergo a follow-up MRI in three months. In August 2020, he was admitted to the urology department since the tumor in the perineum had been enlarged, and the pain had been increased. The patient lost about 10 kg in three months. A new MRI revealed that the tumor had reached 91 × 112 × 70 mm (Figure 4); pathological fracture of right ischium, infiltration of left ischium, and pelvic floor muscles, enlargement of lymph nodes till 15 × 24 mm on right side, and 13 × 23 mm on the left were also visible in the new MRI (Figure 5). An open biopsy of the tumor was performed and the pathology report showed keratotic squamous cell carcinoma in the specimen. The patient, that was already in bed due to weakness and severe pain, was referred to an oncologist for palliative treatment, but his general condition deteriorated rapidly. He died in October 2020 due to the progression of cancer.
2.4. Patient 4 (SZ. W. 43y)
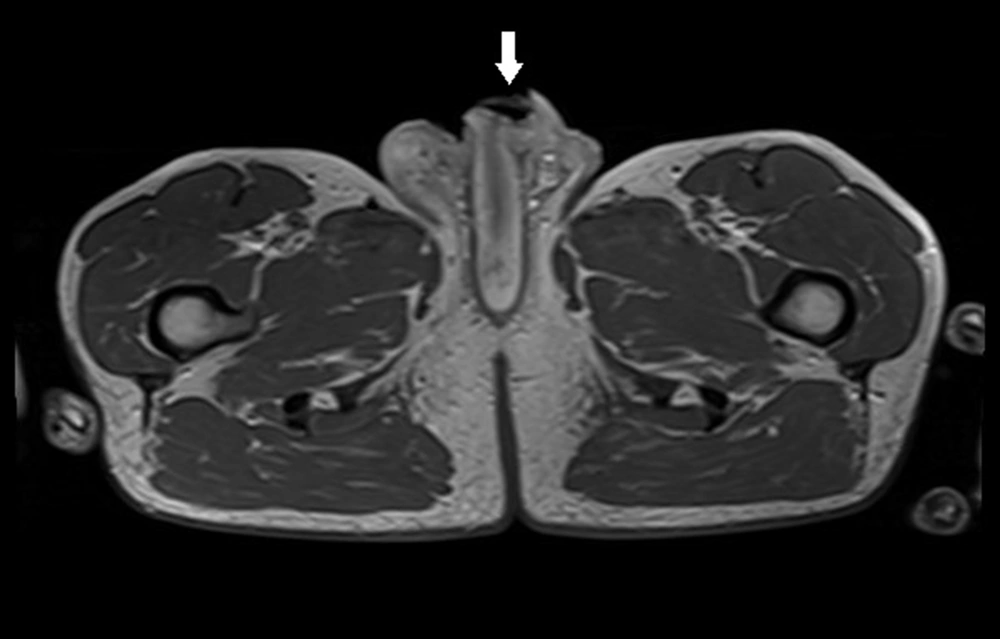
The patient was referred to the urology department in October 2019 for the diagnosis of a vast urethro-cutaneous fistula in the penile scrotum angle. The patient was only diagnosed with chronic obstructive pulmonary disease in a medical interview, but he was not on medications. He underwent several biopsies and resection of lesions in a local hospital in 2019. Squamous papilloma was found in all samples. The infection of Pseudomonas aeruginosa 10/6 and Klebsiella pneumonia 10/6 were confirmed in the urine culture. An open biopsy and urethrocystoscopy were performed in our department, and two tumors were located in the urethra (i.e., one in the distal part, 2 cm from the external orifice; and the second in the bulbar urethra). Foley catheter 16 F was inserted after endoscopic resection. Pathology examination showed squamous cell carcinoma with partially inverted growth. MRI was performed due to diagnostic uncertainty, and infiltration at the base of the penis involving skin, urethra, and corpus spongiosum at the site of the urethral fistula was detected (Figures 6 and 7). In scrotum on right side was located pathologic tumor 29 × 17 × 15 mm - probably inflamed epididymis. Inguinal nodes were enlarged to 23 mm on both sides. The following admission was in December 2019. Upon admission, urethrectomy with resection of the involved skin, corpus spongiosum, and partial scrotum was performed with perineal urethrostomy. The pathology report revealed carcinoma planoepitheliale keratodes invasivum grade 1, growing based on the genital warts with HPV infection. The proximal margin of resection was positive; therefore, the patient was invited for further hospitalization. In February 2020, the distal part of the urethra with a small, firm tumor in the urethrostomy was resected. Cystoscopy was performed, and no evidence of bladder tumor was found; however, samples from bladder were taken. Pathology examination detected condyloma in samples from the urethra, but no other suspected lesions were found in the bladder. The patient continued his everyday life but remained under control. No signs of recurrence or dissemination of the cancer were observed.
3. Discussion
Urethral cancer is one of the rarest neoplasms in males. It accounts for less than 1% of malignancies in men (4, 5). Primary publications on urethra cancer are mainly case reports or case series. Janisch et al. (1) and Rabbani (7) had just completed their studies with a larger population of patients before the present study. The study by Janisch et al. (1) was based on the analysis of several previously published studies examining 7853 male and female patients with urethral cancer. The study by Rabbani (7) also investigated 2724 men with UC whose data were available in the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database. Gillitzer et al. (6) from Maniz reported a single-institution experience. This large urological center had treated nine patients in the previous 20 years. Larger studies were multicenter works that confirmed the rarity of UC. Cancer of urethra can appear in various histopathological forms. Three types of epithelium seen in the urethra lumen can be the sources of cancer. These include (1) in the distal part - meatus and fossa navicularis are present stratified squamous epithelium; (2) in the penile - bulbar and membranous urethra- columnar epithelium; and (3) in the prostatic part - transitional epithelium. Cribriform adenocarcinoma origins from prostate gland (5). All types of epithelia can be a source of cancer. Various histopathological findings have been revealed, but the overlapping of inflammatory lesion makers make the assessment even more difficult. The lymphatic drainage flows through two main ways depending on the distal or proximal part. Penile and bulbar fragments are drained to the superficial and deep inguinal nodes. Lymph flows by pelvic nodes from the membranous and prostatic the urethra to presacral and internal iliac nodes. The direction of the tumor spread by the lymphatic vessels depends on the location of the primary tumor. The cancers in the anterior urethra spread to inguinal nodes, and the cancers in the posterior urethra spread to pelvic lymph nodes. The main symptoms of urethral cancer are associated with difficulty in emptying the bladder (4, 7). A late diagnosis, however, is often established due to assigning this symptom to BPH, which is a prevalent condition in this age group. Bleeding and palpable pathological mass are associated with more advanced diseases. Medications are initially prescribed for the BPH, and only the lack of improvement and appearance of additional symptoms cause an extension of the diagnostic test. This causes additional tardiness in diagnosis and treatment. Reaching an accurate diagnosis requires performing imaging tests and urethrocystoscopy with biopsy. Cystoscopy should also be performed because it can role out the presence of coexisting bladder tumor (1, 5). MRI, as the most precise tool in the assessment of local advancement and a more effective tool than CT, is highlighted in the studies by Janisch et al. (1), Dayyani et al. (4) and Traboulsi et al. (5). Both methods, apart from the primary tumor assessment, visualize the surrounding lymph nodes and organs (1, 4, 5). It should be noted that the enlarged focal nodes are usually metastatic in the case of UC, while they are often inflammatory and reactive in penile cancer. Medical statistics have shown that 30% of the patients have involved lymph nodes at the time of diagnosis (4). In the small series presented in this study, patients nr 1 and 3 had nodal metastasis, patient nr 4 had reactive, inflammatory nodes due to the presence of infection and urinary fistula, and patient nr 2 had no enlarged nodes according to MRI results at the time of diagnosis. Treatment options for UC are surgery, chemotherapy, and radiotherapy, or combinations thereof. The most important clinical prognostic factors are stage, type of cancer, and location of the cancer (1, 4, 7). Low malignancy anterior urethral tumors can only be treated surgically. Reports have suggested that the usual 2 cm margin can be safely reduced to 1 and even to 0.5 cm without losing oncological control (5). Superficial urethral cancer of the prostatic urethra can only be treated by TURP (1), and stromal invasion requires radical urethrectomy. Advanced lesions, regarded as T2-T4 and high-grade tumors, require multimodal treatment. The radical surgery includes cystoprostatectomy, uretrectomy, and penectomy, as well as regional lymphadenectomy (1). In our material, patient nr 1 and patient nr 4 underwent total penectomy and partial penectomy, respectively. The other two patients did not undergo an operation but had biopsies to establish the diagnosis and then received a neoadjuvant the chemotherapy due to the advanced stage of the disease. Chemotherapy can be used as a neoadjuvant or adjuvant. Its composition differs depending on the histological type. Dayyani et al. from MD Anderson Cancer Center used the scheme with cisplatin, gemcitabine, and ifosfamide for dealing with squamous cell carcinomas, but they used the standard MVAC scheme (methotrexate, vinblastine, doxorubicin, and cisplatin) for treating urothelial cancers. They also used 5-fluorouracil, gemcitabine, and cisplatine for dealing with adenocarcinomas and reported treatment response in 72% of the patient after neoadjuvant CHT (4). In this study, two patients underwent neoadjuvant chemotherapy based on cisplatin and gemcytabin, but partial short-term remission and, unfortunately, further progression occurred after a short period. Patient nr 1 had penectomy after CHT, but patient nr 2 received CHT and radiotherapy as the only treatments due to an advanced stage and poor response. Radiotherapy is mainly used as a component of combined therapy. Data on radiation monotherapy are not sufficient and promising (5). Radiotherapy after surgery, along with chemotherapy, is a widely accepted method. The introduction of new devices and technologies in radiotherapy improves the results of XRT and reduces potential radiation complications. It should be noted that radiotherapy contributes significantly to the symptomatic treatment of patients with locally advanced cancer and to the prevention of unresectable recurrences.
3.1. Conclusions
The presented case series was small since only four patients were treated in the previous two years. The low number of patients admitted at the largest university hospital in the region was indicative of the rare occurrence of UC. The patients were already referred from nearby regional hospitals and were reported to specialists in specific symptoms; however, they were subjected to inaccurate diagnosis and treatment for a long period. All patients were accurately diagnosed at our department only when the disease had reached an advanced stage. Previous biopsies had not revealed the true nature of lesions in the case of three patients, which may have been due to the lack of experience in collecting sufficient, deep, and extensive material, and its histopathological assessment. The ultrasound examination (i.e., sonourethrography) was performed only in specialized centers. The US of the urethra was found to be a cheap and non-invasive examination that may have been performed on an outpatient basis and proven very helpful in establishing the diagnosis of PUC. The popularity of this technique and its more comprehensive application may have facilitated the diagnosis, especially when MRI was unavailable and costly. According to epidemiological data, contact with oncogenic HPV may have spread the disease rapidly in the following few decades. Therefore, it was recommended that the practitioners should be prepared for the significant increase in PUC.
The presentation of urethral cancer cases may have brought this rare disease to doctors’ attention and reminded them of the possibility of its development in the differential diagnosis. Urethral cancer was determined as an aggressive disease and its late diagnosis was found to be associated with an unfavorable prognosis despite the inclusion of additional radio and chemotherapy methods. The delay in diagnosis was identified as a key prognostic factor.