1. Background
Primary monosymptomatic nocturnal enuresis (PMNE) is defined as the repeated voiding of urine during bedtime for at least 3 consecutive months in 5-year-old children who have not been dry for > 6 months. It is the most common pediatric urological developmental disorder, which occurs in up to 20% of children aged 5 years and is 3 times more common in boys than girls (1, 2)
Different pathogenic mechanisms have been recognized in children with PMNE, including (1) nocturnal polyuria with sleep urine volume > 35% of the total 24-hour urine output, secondary to the insufficient nocturnal increase of serum antidiuretic hormone (ADH); (2) decreased nocturnal functional bladder capacity; (3) primary sleep disorders; and (4) mixed nocturia (3, 4)
Serum ADH decreases urine volume excretion by increasing water reabsorption from the distal nephron, especially during bedtime. Lines of evidence suggest central nervous system dysfunction in ADH mechanism in children with primary PMNE (5, 6).
Desmopressin (DDAVP), 1-deamino-8-D-arginine vasopressin, is a synthetic analog of arginine vasopressin (AVP) with a more potent antidiuretic effect than AVP. It has been shown to be an effective treatment of nocturnal polyuria by decreasing nocturnal urine production (3, 7).
About 60% - 70% of children with PMNE have a complete or partial response to DDAVP, which is most pronounced in children older than 8 years with monosymptomatic nocturnal polyuria, normal bladder capacity, and less frequent bed-wetting without daytime symptoms (5). Various pharmaceutical formulations of DDAVP have been commercialized, including nasal spray, nasal drops, and oral tablets (7).
DDAVP is a generally effective, safe, and well-tolerated drug. However, hyponatremia has been suggested as a potentially serious adverse effect of DDAVP 14 days or less after the beginning of the treatment (3, 4, 6-8) Hyponatremia/water intoxication might occur in 12% - 22% of patients, more common after intranasal treatment. Therefore, monitoring of serum sodium (Na) has been suggested for the prevention of hyponatremia in some studies (5, 8, 9).
2. Objectives
According to the controversial reports of hyponatremia, this study was performed to identify the incidence and risk factors of hyponatremia in children treated with oral or intranasal DDAVP.
3. Methods
A total of 201 patients with PMNE were enrolled in this cross-sectional study from 2019 to 2021. The Ethics Committee of Ahvaz Jundishapur University of Medical Sciences approved this study (code: IR.AJUMS.REC.1398.906. Link: ethics.research.ac.ir/EthicsProposalView.php?id=121453). Data were extracted from the pediatric professional thesis (license code: CRD-9805), and informed consent was obtained from the children’s legal guardians at the time of the project.
3.1. Inclusion Criteria
Inclusion criteria included patients < 19 years old with primary enuresis of 2 or more wet nights per month who had not been dry for > 6 months after the fifth year of life.
3.2. Exclusion Criteria
Patients with urinary tract infection, congestive heart failure, hepatic disorder, urinary tract obstruction, diuretic use, primary hyponatremia, any underlying disorder with polyuria, and incomplete follow-up were excluded.
All patients were divided in 2 groups using a standard dose (10 - 20 µg) of intranasal DDAVP (n = 127) or oral melt (0.2 mg) of DDAVP (n = 74) before bedtime. Totally, DDAVP was administered for about 6 months, which decreased in a few cases with the early and well-preserved response. Serum Na was measured before (Na1), after 1 month (Na2), and during 2 months of treatment (Na3). Hyponatremia was defined as mild (130 - 135 mEq/L), moderate (120 - 130 mEq/L) or severe (< 120 mEq/L) decreased serum Na concentration.
Treatment efficacy was defined as:
In which pretreatment enuresis was the frequency of wet nights per month at the beginning of treatment, and post-treatment enuresis was defined as the frequency of wet nights after 1 month of treatment with DDAVP. Variables such as age, gender, weight, and frequency of enuresis were evaluated.
3.3. Statistical Analysis
Data analysis was performed using SPSS version 22 (SPSS Inc, Chicago, Ill, USA).
Quantitative and qualitative variables were expressed as mean ± SD, median, frequency, or percentile, respectively. Normality was examined by the Kolmogorov-Smirnov test and Q-Q diagram. Normally distributed continuous variables were assessed by independent sample t test, whereas the Mann-Whitney U test was used for the evaluation of non-normal continuous variables. Predictive factors of hyponatremia were assessed by univariate and multivariate analysis. Univariate analysis was performed by the statistical t test, analysis of variance (ANOVA), chi-square, and Pearson correlation. Linear regression and logistic regression were used for multivariate analysis. P values < 0.05 were considered statistically significant.
4. Results
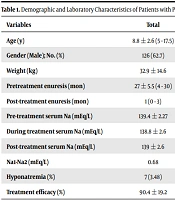
A total of 201 children were included in this study. Males outnumbered females (M/F (126/75) = 1.68). The mean age at the time of the study was 8.8 ± 2.6 (5 - 17.5) years. There was no significant difference in age, gender, body weight, and frequency of pretreatment enuresis between the 2 groups. PMNE significantly improved after oral treatment than intranasal treatment (P = 0.024; Table 1).
| Variables | Total | Oral Desmopressin (n = 74) | Intranasal Desmopressin (n = 127) | P Value |
|---|---|---|---|---|
| Age (y) | 8.8 ± 2.6 (5 - 17.5) | 8.98 ± 2.7 | 8.7 ± 2.4 | 0.51 |
| Gender (Male); No. (%) | 126 (62.7) | 50 (67.6) | 76 (59.8) | 0.27 |
| Weight (kg) | 32.9 ± 14.6 | 33.4 ± 13.6 | 32.6 ± 15.2 | 0.53 |
| Pretreatment enuresis (mo) | 27 ± 5.5 (4 - 30) | 26.8 (5.4) | 27.5 (5.6) | 0.72 |
| Post-treatment enuresis (mo) | 1 (0 - 3) | 0 (0 - 2) | 1 (0 - 3) | 0.032 |
| Pre-treatment serum Na (mEq/L) | 139.4 ± 2.27 | 139.1 ± 2.2 | 139.6 ± 2.2 | 0.14 |
| During treatment serum Na (mEq/L) | 138.8 ± 2.6 | 138.6 ± 2.5 | 138.8 ± 2.6 | 0.62 |
| Post-treatment serum Na (mEq/L) | 139 ± 2.6 | 139.25 ± 2.42 | 138.9 ± 2.7 | 0.61 |
| Na1-Na2 (mEq/L) | 0.68 | 0.5 | 0.79 | 0.52 |
| Hyponatremia (%) | 7 (3.48) | 3 (4.05) | 4 (3.15) | 0.73 |
| Treatment efficacy (%) | 90.4 ± 19.2 | 94.6 ± 11.5 | 88.1 ± 22.3 | 0.024 |
Demographic and Laboratory Characteristics of Patients with Primary Monosymptomatic Nocturnal Enuresis
Treatment efficacy (50% or more reduction of wet nights/after a month of treatment) was 100% in 93 (46.2%) cases, and more than 90% in 157 (78.1%) patients, which was higher in oral treatment than in intranasal treatment (P = 0.01, P = 0.028; Table 2). In addition, oral treatment was observed to be easier to administer with better cooperation compared to intranasal treatment.
| Variables | Total | Oral Desmopressin (n = 74) | Intranasal Desmopressin (n = 127) | P Value |
|---|---|---|---|---|
| Complete cure (100%) | 93 (46.2) | 50 (39.3) | 43 (48.1) | 0.01 |
| Efficacy (> 90%) | 157 (78.1) | 93 (73.2) | 64 (86.4) | 0.028 |
| Efficacy (30% - 90%) | 34 (16.9) | 24 (18.8) | 10 (13.5) | 0.32 |
Therapeutic Efficacy of DDAVP in Children with Primary Monosymptomatic Nocturnal Enuresis a
There was no significant difference between the 2 groups in initial (P = 0.14) and post-therapeutic (P = 0.62) serum Na. In addition, no significant correlation was found between pre- and post-therapeutic serum Na with age, gender, body weight, and treatment efficacy (P > 0.05; Table 2).
Mild hyponatremia (132 - 134 mEq/L) occurred in 7 (3.5%) patients (3 in oral treatment and 4 in intranasal treatment; P = 0.73), with no significant correlation to age, gender, body weight, frequency of enuresis, initial serum Na, and oral or intranasal treatment. Serum Na decreased > 5 mEq/L in 16 patients, irrespective of age, gender, body weight, frequency of enuresis, and mode of treatment (oral vs intranasal). Of them, hyponatremia occurred in 5 cases (P < 0.001). Therefore, decreased serum Na > 5 mEq/L was a significant risk factor for the prediction of hyponatremia in children who received DDAVP (Table 3).
| Variables | Normal Na | Hyponatremia | P Value |
|---|---|---|---|
| Age (y) | 8.85 ± 2.58 | 8 ± 2.7 | 0.43 |
| Weight (kg) | 33 ± 14.6 | 28 ± 14 | 0.4 |
| Pre-treatment serum Na (mEq/L) | 139.48 ± 2.27 | 139.42 ± 2.22 | 0.952 |
| Post-treatment serum Na (mEq/L) | 138.99 ± 2.39 | 133.14 ± 0.89 | < 0.001 |
| Na1-Na2 (mEq/L) | 0.48 ± 3 | 6.24 ± 2.49 | < 0.001 |
| > 5-mEq/L decreased serum Na; No. (%) | 2 (28.6) | 5 (71.4) | < 0.001 |
Serum Sodium Alterations in Primary Monosymptomatic Nocturnal Enuresis During Treatment with DDAVP
Post-therapeutic serum Na was significantly lower in hyponatremic patients (P < 0.001). In addition, the mean difference between pre-and post-therapeutic serum Na was significantly higher in the hyponatremia group (P < 0.001).
In addition, headache and nose itching was reported in a few patients in the intranasal group.
5. Discussion
Since the 1980s, intranasal DDAVP has been an effective treatment in children with PMNE. If used correctly, it is a generally safe and well-tolerated drug in the majority of patients, irrespective of age and gender (3, 5). PMNE cured or improved in 46% and 78% of our patients, respectively, with more therapeutic effect in oral treatment than in intranasal treatment.
Similar to our study, Lee et al. indicated that oral DDAVP was an effective treatment of PMNE by reducing nocturnal urine volume and increasing nocturnal bladder capacity, with no significant changes in Na, potassium, creatinine, and body weight (3).
DDAVP has been shown to have a more beneficial impact on children with PMNE than children without PMNE, with the most ADH deficiency and increased Na excretion (10). An acceptable response to DDAVP was reported with all different doses by Schulman et al. (11).
Hyponatremia/water intoxication is a rare but potentially serious adverse effect of DDAVP in both children and adults (3, 6).
Both oral and intranasal DDAVP treatments had no significant effect on serum Na in our study, and post-therapeutic serum Na had no significant difference between the 2 groups in initial Na. However, serum Na decreased > 5 mEq/L in 16 patients, and hyponatremia occurred in 5 of them, which was a significant risk factor for the prediction of hyponatremia. Totally, hyponatremia occurred significantly in patients with a > 5-mEq/L decreased serum Na after treatment of PMNE (P < 0.001).
In a meta-analysis, hyponatremia was reported in 7.6% of patients. However, decreased serum Na did not occur in some of the patients who received DDAVP at least 10-fold higher than the recommended dose (3).
Davidson et al. reported mild and severe hyponatremia in 74% and 9% of children who received DDAVP, respectively. However, none of them had significant complications of decreased Na (12). Hyponatremia occurred in 3.5% of our patients. The majority of them were asymptomatic and identified incidentally during serum Na measurement.
Although a large number of hyponatremic patients are asymptomatic (4, 8); however, mild manifestations of hyponatremia, such as nausea, vomiting, and headaches, might occur during the first 2 weeks of treatment (prodromal phase) and should be promptly assessed. In addition, severe symptoms, such as hyponatremic encephalopathy, cerebral edema, seizures, and brainstem herniation, might not recognize until late in hyponatremia, resulting in a clinical challenge (6, 8, 13). Clinical manifestations of hyponatremia are more prevalent during the large or rapid deterioration of serum Na, which might occur any time after the beginning of treatment (mostly during the first 14 days). However, severe signs of hyponatremia might occur in 10% 1 year or more after the beginning of treatment (14).
Both old and young age at treatment (not recommended in < 5 years old), low initial serum Na, high initial urine volume, excessive fluid intake, weight gain, high dose of DDAVP, intranasal preparation at the beginning of treatment, and concomitant drug or illness affecting fluid balance (vomiting, diarrhea, hepatic disease, surgery, stress, pain, renal disorder, headache, and imipramine) have been considered as the risk factors of hyponatremia (4, 5, 8, 9, 15). Monitoring serum electrolytes and urine output at 15-20 hours after treatment is recommended in children < 2 years of age, intercurrent illness, alteration of hydration, and intranasal treatment longer than 1 week (16, 17)
According to the International Children's Continence Society (ICCS) recommendations, stopping drinking 2 hours and using DDAVP 1 hour before bedtime are recommended to decrease the risk of hyponatremia.
The antidiuretic effect of DDAVP lasts for 1 night or less. Therefore, discontinuation of DDAVP may be sufficient for maintaining normal water and electrolyte values, in addition to fluid monitoring, if headache, nausea, or vomiting occurs (3, 5).
Children who received oral treatment had less hyponatremia than intranasal treatment, although it was not significant in our study. Ramakrishnan indicated that oral treatment was a safer alternative than intranasal treatment for its low bioavailability, in addition to the predictable and practical duration of action (5)
Our study had some limitations as follows: (1) the low number of children with hyponatremia (accordingly, multicentric studies with a higher number of children are recommended to achieve more comprehensive results); (2) the high cost of melt formula that limited its use; and (3) the absence of a healthy control group without DDAVP administration. However, we used a laboratory normal range of serum Na (135 - 150 mEq/L) for the definition of hyponatremia (serum Na < 135 mEq/L).
The results of this study indicate that (1) DDAVP is a safe and effective treatment of PMNE with a low complication rate; (2) oral DDAVP is more efficient than intranasal treatment; (3) hyponatremia is a rare and asymptomatic complication of DDAVP, and routine monitoring of serum Na is not suggested in all children receiving DDAVP, except for at-risk and symptomatic patients; (4) decreased serum Na > 5 mEq/L is a warning sign of developing hyponatremia in children receiving DDAVP.