1. Background
Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) is a novel respiratory disease responsible for the most recent pandemic, which has imposed a heavy burden on almost every society across the globe (1). Although estimating the actual fatality rate of Coronavirus disease 2019 (COVID-19) is complicated and varies from place to place, a recent meta-analysis reported an infection fatality rate of 0.86% (2). The highest mortality rate has been reported in western countries, and 20.5 million years of life have been lost to this disease globally (1, 3). The disease is manifested by acute respiratory symptoms with variable severity and outcome (1). It has been demonstrated that most COVID-19 cases are asymptomatic or mildly symptomatic (1). The majority of patients with mild symptoms develop fever, dry cough, fatigue, and pneumonia with ground-glass opacities in their lung fields (4). The more severe cases account for up to 14% of SARS-CoV-2-infected patients who develop severe dyspnea requiring hospitalization and admission to intensive care units (ICUs) (4).
Less than 5% of patients experience critical illness and develop acute respiratory distress syndrome and multiorgan failure (4). Like many other infectious diseases, COVID-19 has specific risk factors predicting disease severity and patient outcomes (4). The SARS-CoV-2 infection has affected specific populations differently from the early months of the pandemic. Among different populations, the elderly, especially those older than 65, are more susceptible to this disease and die more frequently (1). Most of these individuals experience various comorbidities, including malignancies and respiratory diseases (1).
Aside from age, male gender, obesity, and some comorbidities, including diabetes, hypertension, and cardiovascular disease, have been reported as common risk factors for severe COVID-19 (5). Alongside these risk factors, numerous laboratory markers have been introduced to predict disease severity. Increased cardiac troponin I/T and liver enzymes, elevated inflammatory markers, including C-reactive protein, and decreased lymphocytes have been identified as the markers of severe SARS-CoV-2 infection (5). Unlike these serum markers, variations in serum electrolytes have not been widely studied in COVID-19, and their relationships with disease severity are still unclear.
A recent study by Nahkuri et al. suggested electrolyte abnormalities as an essential mechanism during COVID-19 (6). Angiotensin-converting enzyme 2 (ACE-2), the entry point of SARS-CoV-2, is an antagonist of ACE-regulating electrolyte hemostasis (6). The SARS-CoV-2 invasion affects the bioavailability of the ACE receptor, downregulating the ACE-2 expression (6). Dysregulated expression of ACE-2 affects angiotensin II, a primary bioactive product of the rennin-angiotensin system, regulating electrolyte hemostasis (6). Therefore, recent clinical studies have tried to establish a link between possible electrolyte imbalance and COVID-19 severity. Among serum electrolytes, five electrolytes, including calcium, sodium, phosphorus, potassium, and magnesium, are the most important. Besides the possible adverse effects of SARS-CoV-2 on electrolyte balance, abnormal levels of these electrolytes have been reported in other diseases. Decreased phosphorus level has been observed in respiratory diseases, including chronic obstructive pulmonary disease (COPD) and acute respiratory failure (7, 8). Similarly, hypocalcemia and low serum magnesium level have been linked to an acute exacerbation of COPD and respiratory infection (9, 10).
2. Objectives
Regarding the possible link between abnormal serum electrolyte levels and adverse pulmonary function, and the possible adverse effects of SARS-CoV-2 infection on electrolyte hemostasis, the present study aimed to assess the frequency of abnormal electrolyte levels and its relationship with COVID-19 severity in hospitalized COVID-19 patients.
3. Methods
This retrospective clinical study was approved by the Mashhad University of Medical Sciences Ethics Committee (IR.MUMS.MEDICAL.REC.1399.514) and conducted in Imam Reza Hospital (Mashhad, Iran). The medical records of every COVID-19 patient admitted to the Emergency Department of Imam Reza Hospital (May - August 2020) were evaluated, and every patient with a confirmed diagnosis of COVID-19 based on reverse transcription-polymerase chain reaction (RT-PCR) was included in the study. Patients who had incomplete medical records were excluded from the study. Patients' demographic data, including gender, age, underlying diseases, substance abuse, and clinical findings upon admission, were collected. Disease severity was evaluated based on oxygen saturation (pulse oximetry), lung involvement severity in computed tomography (CT) scan, inflammatory serum biomarkers, and admission to the intensive care unit (ICU). Five serum electrolytes, including sodium, potassium, magnesium, calcium (corrected by serum albumin level), and phosphorus, were documented for every patient.
The COVID-19 severity was assessed based on the World Health Organization (WHO) criteria, including oxygen saturation and respiratory rate. Based on the WHO criteria, a respiratory rate above 30/min or oxygen saturation below 93% are the criteria for severe COVID-19 (11). Since a CT scan score ≥ 18 could be associated with increased mortality risk (12), study variables were analyzed based on the CT score cutoff. The arterial PO2 to the fraction of inspired oxygen ratio (P/F) could not be calculated since the arterial blood gas was performed not for all patients.
3.1. Statistical Analysis
Data were analyzed in SPSS software (version 16), and a p value less than 0.05 was considered statistically significant. The normality of continuous data was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Mean, and standard deviation (SD) were used for normally distributed variables, while median and interquartile ranges (IQR) were utilized for non-normally distributed variables. The independent t-test or Mann-Whitney test was employed to compare the continuous variables between the groups. Frequency and percentage were used for categorical variables. The comparison of categorical variables between the groups was performed using the chi-square or Fisher's exact test. The relationship between COVID-19 severity and study variables was assessed using a generalized estimation equation (GEE).
4. Results
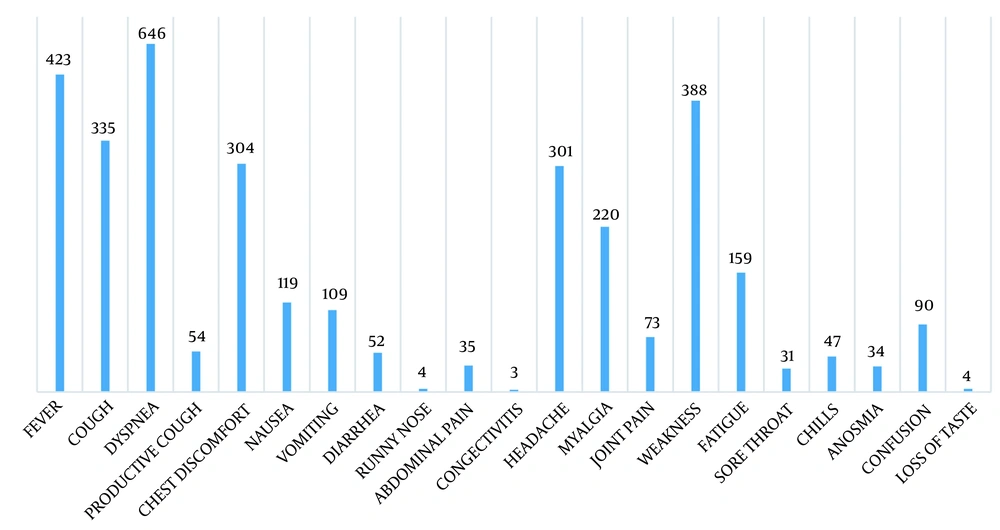
The majority of patients (60%) were male, and the mean age of the total population was 58.87 ± 16.81 years. Most patients were hypertensive (52.3%) or diabetic (40.2%) (Table 1). The most common clinical symptom was dyspnea, observed in 464 patients (Figure 1). Severe COVID-19 infection was detected in 535 (94.9%) patients. The comparison of demographic and laboratory variables between severe and non-severe COVID-19 patients is presented in Table 2.
| Variables | Total (N = 564) | COVID-19 Severity | P Value | |
|---|---|---|---|---|
| Non-severe (n = 23) | Severe (n = 535) | |||
| Age (y) | 58.87 ± 1.82 | 51.74 ± 17.84 | 59.19 ± 16.64 | 0.037 b |
| Gender | 0.436 | |||
| Male | 334 (60.0) | 12 (52.2) | 322 (60.3) | |
| Female | 223 (40.0) | 11 (47.8) | 212 (39.7) | |
| Diabetes | 225 (40.3) | 4 (17.4) | 221 (41.3) | 0.022b |
| Hypertension | 292 (52.3) | 7 (30.4) | 285 (53.3) | 0.032b |
| Asthma | 13 (2.3) | 0 (0.0) | 13 (2.4) | 0.999 |
| Ischemic heart disease | 160 (28.7) | 2 (8.7) | 158 (29.5) | 0.033b |
| Chronic kidney disease | 39 (7.0) | 0 (0.0) | 39 (7.3) | 0.394 |
| Renal transplantation | 10 (1.8) | 0 (0.0) | 10 (1.9) | 0.768 |
| Malignancy | 26 (4.7) | 0 (0.0) | 26 (4.9) | 0.618 |
| Chronic obstructive pulmonary disease | 67 (12.0) | 1 (4.3) | 66 (12.3) | 0.342 |
| Intensive care admission | 143 (26.0) | 1 (4.5) | 142 (26.9) | 0.022b |
| CT score | 12.27 ± 4.95 | 8.57 ± 3.23 | 12.41 ± 4.97 | < 0.001 b |
| Admission duration | 9.00 (6.00) | 7.70 ± 3.43 | 9.00 (6.00) | 0.021 b |
| O2 saturation | 82.74 ± 7.66 | 95.39 ± 1.23 | 82.21 ± 7.36 | < 0.001 b |
| Pulse rate (/min) | 109.51 ± 17.92 | 88.39 ± 13.80 | 110.45 ± 17.52 | < 0.001 b |
| Respiratory rate (/min) | 29.95 ± 6.64 | 21.52 ± 3.80 | 30.31 ± 6.49 | < 0.001 b |
| Systolic blood pressure (mmHg) | 128.40 ± 20.47 | 126.82 ± 23.58 | 128.52 ± 20.33 | 0.702 |
| Diastolic blood pressure (mmHg) | 82.08 ± 11.42 | 81.82 ± 14.68 | 82.13 ± 11.26 | 0.899 |
| WBC (/106) | 8.10 (3.70) | 6.30 (4.32) | 8.10 (3.82) | 0.085 |
| PMN (%) | 79.66 ± 11.33 | 73.59 ± 10.50 | 79.94 ± 11.28 | 0.010 b |
| Lymphocyte (%) | 12.40 (7.40) | 18.89 ± 8.49 | 12.00 (12.00) | 0.008 b |
| FBS (mg/dL) | 112.00 (55.00) | 125.30 ± 51.25 | 112.50 (57.50) | 0.425 |
| Urea (mg/dL) | 40.00 (24.50) | 30.00 (5.00) | 41.00 (25.00) | 0.001 b |
| Creatinine (mg/dL) | 1.00 (0.40) | 0.97 ± 0.19 | 2.58 ± 0.80 | 0.505 |
| Sodium (mg/dL) | 134.98 ± 11.97 | 136.91 ± 3.16 | 134.90 ± 12.26 | 0.433 |
| Potassium (mg/dL) | 4.20 (0.40) | 3.80 ± 0.47 | 4.20 (0.40) | < 0.001 b |
| Adjusted potassium (mg/dL) | 4.61 ± 0.24 | 4.50 ± 0.12 | 4.62 ± 0.25 | < 0.001 b |
| Calcium (mg/dL) | 8.65 ± 0.83 | 8.75 ± 1.01 | 8.65 ± 0.82 | 0.584 |
| Phosphorus (mg/dL) | 3.76 ± 1.90 | 3.74 ± 0.96 | 3.76 ± 1.94 | 0.971 |
| Magnesium (mg/dL) | 2.14 (0.15) | 2.21 ± 0.38 | 2.14 (0.16) | 0.726 |
| LDH (U/L) | 582.00 (216.00) | 468.73 ± 149.47 | 589.00 (221.00) | 0.001 b |
| CPK (U/L) | 97.00 (206.00) | 336.50 (263.50) | 97.00 (203.00) | 0.693 |
| ESR (mm/h) | 50.00 (24.00) | 41.00 (32.50) | 50.00 (25.00) | 0.561 |
| CRP (mg/L) | 87.50 (77.75) | 59.00 (36.75) | 88.95 (67.10) | 0.006 b |
| Death | 133 (24.7) | 1 (4.3) | 132 (25.6) | 0.023b |
Abbreviations: WBC, white blood cell; PMN, polymorphonuclear; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a Values are expressed as mean ± SD or No. (%).
b Significant difference.
| Variables | Prognosis | P Value | |
|---|---|---|---|
| Good Prognosis (n = 490) | Poor Prognosis (n = 69) | ||
| Age (y) | 58.97 ± 17.25 | 58.35 ± 13.29 | 0.773 |
| Gender | 0.565 | ||
| Male | 292 (59.6) | 43 (63.2) | |
| Female | 198 (40.4) | 25 (36.8) | |
| Diabetes | 185 (37.8) | 41 (59.4) | 0.001 b |
| Hypertension | 248 (50.6) | 44 (63.8) | 0.041 b |
| Asthma | 13 (2.7) | 0 (0.0) | 0.386 |
| Ischemic heart disease | 145 (29.6) | 17 (24.6) | 0.396 |
| Chronic kidney disease | 30 (6.1) | 10 (14.5) | 0.012 b |
| Renal transplantation | 9 (1.8) | 2 (2.9) | 0.223 |
| Malignancy | 25 (5.1) | 1 (1.4) | 0.233 |
| Chronic obstructive pulmonary disease | 58 (11.8) | 8 (11.6) | 0.953 |
| Intensive care admission | 95 (19.7) | 47 (69.1) | < 0.001 b |
| Admission duration | 8.00 (5.00) | 15.00 (8.50) | < 0.001 b |
| O2 saturation | 84.04 ± 6.90 | 73.90 ± 6.80 | < 0.001 b |
| Pulse rate (/min) | 107.71 ± 17.97 | 121.25 ± 22.35 | < 0.001 b |
| Respiratory rate (/min) | 29.40 ± 6.77 | 33.59 ± 4.15 | < 0.001 b |
| Systolic blood pressure (mmHg) | 128.55 ± 20.29 | 127.25 ± 22.35 | 0.622 |
| Diastolic blood pressure (mmHg) | 82.03 ± 11.35 | 82.03 ± 12.32 | 0.990 |
| WBC (/106) | 7.90 (3.50) | 10.60 (4.80) | < 0.001 b |
| PMN (%) | 78.74 ± 11.49 | 85.98 ± 7.83 | < 0.001 b |
| Lymphocyte (%) | 13.00 (7.00) | 8.00 (4.00) | < 0.001 b |
| FBS (mg/dL) | 110.00 (51.00) | 124.00 (68.00) | 0.009 b |
| Urea (mg/dL) | 39.00 (25.00) | 49.00 (21.00) | 0.002 b |
| Creatinine (mg/dL) | 1.00 (0.40) | 1.10 (0.30) | 0.360 |
| Sodium (mg/dL) | 134.77 ± 12.71 | 136.58 ± 4.45 | 0.243 |
| Potassium (mg/dL) | 4.20 (0.40) | 4.0 (0.20) | 0.110 |
| Adjusted potassium (mg/dL) | 4.60 ± 0.24 | 4.66 ± 0.24 | 0.056 |
| Calcium (mg/dL) | 8.69 ± 0.84 | 8.39 ± 0.68 | 0.007 b |
| Phosphorus (mg/dL) | 3.77 ± 2.00 | 3.76 ± 1.03 | 0.982 |
| Magnesium (mg/dL) | 2.10 (0.20) | 2.27 ± 0.39 | 0.035 b |
| LDH (U/L) | 560.00 (188.00) | 780.00 (385.50) | < 0.001 b |
| CPK (U/L) | 97.00 (206.00) | 114.00 (729.00) | 0.614 |
| ESR (mm/h) | 49.00 (24.00) | 62.00 (22.50) | 0.023 b |
| CRP (mg/L) | 80.00 (62.00) | 146.60 (104.00) | < 0.001 b |
| Death | 93 (19.7) | 41 (60.3) | < 0.001 b |
Abbreviations: WBC, white blood cell; PMN, polymorphonuclear; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a Values are expressed as mean ± SD or No. (%).
b Significant difference.
There was a significant difference in age (P = 0.037), ICU admission (P = 0.022), CT score (P < 0.001), admission length (P = 0.021), O2 saturation (P < 0.001), pulse rate (P < 0.001), respiratory rate (P < 0.001), polymorphonuclear leukocyte (PMN) percentage (P = 0.010), lymphocyte percentage (P = 0.008), serum urea (P = 0.001), serum potassium (P < 0.001), adjusted serum potassium (P < 0.0001), lactate dehydrogenase (LDH) (P = 0.001), and C-reactive protein (CRP) (P = 0.006) between severe and non-severe COVID-19 patients. Furthermore, there was a significant difference in the prevalence of diabetes (P = 0.022), ischemic heart disease (P = 0.032), and death (P = 0.023) between the severe and non-severe COVID-19 groups.
Based on the CT score cutoff, 69 (12.3%) cases were regarded as poor prognoses. The comparison of study parameters between the prognosis groups is displayed in Table 2. There was a significant difference between the groups in the length of admission (P < 0.001), O2 saturation (P < 0.001), pulse rate (P < 0.001), respiratory rate (P < 0.001), white blood cell (WBC) count (P < 0.001), PMN percentage (P < 0.001), lymphocyte percentage (P < 0.001), fasting blood sugar (FBS) (P = 0.009), urea (P = 0.002), serum calcium (P = 0.007), serum magnesium (P = 0.035), serum LDH (P < 0.001), erythrocyte sedimentation rate (ESR) (P = 0.023), and CRP (P < 0.001). Furthermore, there was a significant difference in the prevalence of diabetes (P = 0.001), hypertension (P = 0.041), chronic kidney disease (CKD) (P = 0.012), ICU admission (P < 0.001), and death (P < 0.001). The GEE analysis pointed to the significant relationship between severe COVID-19 and serum potassium (P < 0.001), LDH (P = 0.015), and ICU admission (P < 0.001) (Table 3).
| Variables | Coefficient | P Value |
|---|---|---|
| Serum potassium | 1.697 | < 0.001 a |
| LDH | 0.003 | 0.015 a |
| CRP | < 0.001 | 0.971 |
| Diabetes | -0.139 | 0.842 |
| Hypertension | 0.228 | 0.823 |
| IHD | 0.728 | 0.446 |
| Age | 0.021 | 0.381 |
| CT score | 0.117 | 0.059 |
| WBC | 0.014 | 0.759 |
| PMN (%) | 0.056 | 0.054 |
| Lymphocyte (%) | 0.031 | 0.380 |
| Urea | 0.004 | 0.606 |
| ICU admission | -1.962 | < 0.001 a |
| Serum sodium | 0.002 | 0.966 |
| Serum calcium | 0.553 | 0.085 |
| Serum phosphorus | -0.021 | 0.675 |
| Serum magnesium | -0.849 | 0.286 |
Abbreviations: LDH, lactate dehydrogenase; CRP, C-reactive protein; IHD, ischemic heart disease; CT, computed tomography; WBC, white blood cell; PMN, polymorphonuclear.
a Significant difference.
5. Discussion
The present study demonstrated that the mean level of five serum electrolytes, including sodium, potassium, magnesium, phosphorous, and calcium, were within the normal range in hospitalized COVID-19 patients. The serum potassium level was significantly higher in the severe group than in non-severe patients. When patients were categorized into good and poor prognosis based on the CT score, serum calcium level was significantly higher in the good prognosis group, and serum magnesium level was significantly higher in the poor prognosis group. The GEE analysis showed a significant relationship between severe COVID-19 infection and serum potassium.
Although the possible mechanism of abnormal serum electrolytes has been suggested by Nahkuri et al., the abnormal levels of serum electrolytes in COVID-19 patients have been addressed in limited studies (6). Among the population of hospitalized COVID-19 patients in the current study, the serum levels of sodium, potassium, magnesium, phosphorous, and calcium were within the normal range on admission. The present research results contrast with those obtained in a previous study that demonstrated hypokalemia as a prevalent finding in COVID-19 patients (13) and that hypokalemia was not correlated with poor prognosis (13). Like hypokalemia, dysnatremia has also been prevalent among hospitalized COVID-19 patients upon admission. Hirsch et al. pointed out that hyponatremia was more prevalent than hypernatremia among COVID-19 patients and linked these electrolyte abnormalities to increased hospital stay (14). They reported that moderate and severe hypernatremia was associated with an increased risk of hospital mortality (14).
Yang et al. addressed similar findings regarding serum calcium and phosphorus levels in moderate and severe COVID-19 patients (15). They demonstrated that low calcium and phosphorus levels were correlated with ICU admission and abnormal lung and liver function (15). Regardless of these findings of the abnormal level of electrolytes in COVID-19 patients, some critical issues should be addressed concerning the controversial results. Various studies used different disease severity scores, and the underlying diseases in COVID-19 patients were considered not in all studies. Therefore, the comparison of study results should be made cautiously.
The present research results align with those reported in the studies with similar methodological criteria. Sarvazad et al. evaluated serum levels of sodium, potassium, and magnesium, regardless of COVID-19 patients with underlying diseases, and demonstrated that these electrolytes were significantly different between ICU-admitted patients and outpatients (16). In a similar vein, in the present, magnesium level was significantly elevated in poor prognosis patients (16). Nonetheless, the present study observed no significant relationship between sodium level and ICU admission.
Regardless of different sample sizes, the current study considered COVID-19 patients with different underlying diseases. Tezcan et al. demonstrated that hyponatremia and hypocalcemia were related to ICU admission and mortality, while neither hypokalemia nor hyperkalemia was linked to these outcomes (17). Moreover, serum LDH levels greater than 240 U/L, D-dimer levels greater than 1000 ng/mL, lymphocyte count of less than 1000/uL, and serum ferritin greater than 300 mg/mL were present among COVID-19 patients with serum electrolyte abnormalities (17). Regardless of similar findings of the serum levels of calcium and potassium in the mentioned study, the present study showed different results regarding sodium levels (17).
Discrepancies between the present study results and those obtained by Tezcan et al. can be attributed to a different grouping of COVID-19 patients (17). Contrary to the present study, Tezcan et al. (17) grouped their study population based on electrolyte abnormalities. A recent review study on 1,415 COVID-19 patients demonstrated that low serum sodium, calcium, and potassium levels were correlated with more severe illness (18). Considering the elevated ESR and CRP levels as indicators of more severe disease, the sodium level was significantly correlated with a more severe infection in the present study.
After adjusting potassium based on serum urea, serum potassium was still higher in patients with severe COVID-19 than in non-severe COVID-19 patients. In contrast, the difference in serum potassium was no longer significant between good and poor prognosis groups after adjusting for serum urea. This finding contrasts with the study results by Lippi et al. (18). Different criteria for severe illness could explain the possible differences among studies. Other studies regarded severe illness as requiring mechanical ventilation and ICU admission; however, in the present study, severe illness was defined based on oxygen saturation and respiratory rate (18).
The increased serum potassium level in severe COVID-19 patients might be attributed to acute kidney injury (19). In the present study, serum urea but not creatinine was significantly higher in severe COVID-19 patients than in non-severe COVID-19 patients. This finding might indicate some degrees of kidney injury in severe COVID-19 patients. Nevertheless, the difference persisted after adjusting serum potassium for serum urea level. This finding may point to another possible mechanism for increased potassium in severe COVID-19 patients. Since the present study was conducted based on a cross-sectional design, the patients' data regarding changes in kidney function tests were not available.
Another possible reason for increased serum potassium is cell lysis. It was previously hypothesized that cytokine storm might result in tumor lysis syndrome in patients with COVID-19, associated with increased uric acid, LDH, lactate, and acute kidney injury (20). Similarly, serum LDH was higher in severe COVID-19 patients in the present study. Therefore, tumor lysis syndrome has possibly occurred in some patients; nonetheless, the condition has not reached kidney injury at the time of electrolyte assessment.
5.1. Study Limitations
One of the significant limitations of the present study could be the time allocated to assessing serum electrolytes. Future studies should assess electrolyte abnormalities serially and more than once during hospital admission. Another limitation of the study was the high proportion of patients with severe COVID-19 infection. Due to the high incidence of the disease, most of the patients admitted to the hospital had severe symptoms. Nevertheless, the GEE analysis was performed to reduce the effect of missing data and sample size differences on the findings. Therefore, there is a need for studies that include an equal number of patients with different severity of the illness to assess the present study's results.
5.2. Conclusions
As evidenced by the present study results, serum electrolyte abnormalities were correlated with COVID-19 severity. Among five different electrolytes, including sodium, potassium, magnesium, calcium, and phosphorus, the calcium level was a more reliable indicator of severe disease, correlating with severe disease biochemical and imaging markers. In contrast to calcium, serum phosphorus level was less likely to be correlated with severe disease.