1. Background
Interstitial cystitis (IC)/bladder pain syndrome (BPS) is a chronic bladder disease characterized by bladder pain and lower urinary tract symptoms that have lasted for more than six weeks in the absence of other identifiable causes (1). With a mean age of about 40 years and its debilitating symptoms (e.g., urgency, frequency, nocturia, dysuria), it can significantly decrease patients' quality of life. Its prevalence has been reported as approximately 1% - 2 % (2), but it is probably underdiagnosed due to the absence of clear diagnostic criteria. Hydrodistention of the bladder with the patient under anesthesia is usually used for diagnostic purposes and is often the first therapeutic procedure employed (3). However, poor long-term results have limited the use of hydrodistention as a treatment option (4). The exact pathophysiology behind IC/BPS remains unknown. Parsons and Hurst hypothesized that IC could result from glycosaminoglycan (GAG) dysfunction in the urothelium (5). GAGs are located on the luminal surface of the urothelium, and they protect the urothelium from the adherence of pathogens (6). In addition, GAGs prevent urine absorption by forming a mucus barrier between urine and the urothelium (6), making the urothelium the most impermeable membrane in the mammalian body (7). Given these facts, using exogenous GAG may be beneficial for treating IC/BPS (8). Hyaluronate is one of the main components of the GAG layer of the bladder (9). Intravesical sodium hyaluronate can temporarily replace the bladder's disrupted GAG layer and ameliorate IC symptoms (10). In this regard, Hüther et al. evaluated the role of hyaluronic acid in the treatment of IC in an in vitro study. Following inflammation induction by tumor necrosis factor (TNF), 0.8 mg/mL hyaluronic acid treatment resulted in a 24 - 48 h increase in GAG synthesis and a decrease in inflammatory cytokine levels (11).
Previous studies confirm these findings as a 30% and 71% long-term response to treatment with intravesical hyaluronic acid has been reported (12, 13). Shao et al. (2010) compared the effects of hydrodistention plus intravesical hyaluronic acid, hydrodistention plus intravesical heparin, and hydrodistention alone in patients with severe IC and bladder capacity of fewer than 200 mL. They observed significantly better improvements in the hyaluronic acid group (3).
2. Objectives
In light of these findings, we aimed to compare the short-term treatment of IC/BPS by bladder hydrodistention plus intravesical sodium hyaluronate (Cystistat®) and hydrodistention alone.
3. Methods
This randomized controlled clinical trial was conducted in the Urology Clinic of Imam Reza Hospital in 2017. This clinic is the referral center for IC/BPS patients in Khorasan Province, Iran.
Twenty-four female IC/BPS patients were enrolled by convenience sampling using the IC diagnostic questionnaire and clinical examination according to the international continence society definition of IC/BPS (14). To avoid confounding, patients were carefully selected. The patients were older than 18 years old, and all had previously been treated with a daily dose of 25 mg of amitriptyline for at least one month and did not show any improvement in symptoms. The exclusion criteria entailed having a history of bacterial cystitis in the last three months, active genital herpes, history of vaginal cervical or uterine cancer, diverticulum, taking cyclophosphamide, bladder tuberculosis, abdomen or pelvis radiotherapy, symptoms of vaginitis, bladder stones, symptom relief with antimicrobials or anticholinergics, frequency of urination less than eight times a day, and benign or malignant bladder tumors.
The frequency subscale in two groups was used to calculate the sample size according to the study of Shao et al. (2010) (3). The mean score of urinary frequency in the intervention and control groups was 14.6 ± 3.5 and 19.7 ± 3.8, respectively. The sample size was calculated to be 10 for each group with α = 0.05, β = 0.2, and 0.84 test power using PASS software. Considering 20% lost to follow-up, 12 patients were eventually assigned to each group.
Hydrodistention and hydrodistention plus cystistat® were performed weekly for four weeks, followed by monthly until two months later (six sessions in total). To evaluate the effects of the treatment, all patients completed the O`Leary-Sant IC questionnaire (15) just before starting the intervention, as well as one week, one month, and three months after the last intervention. The Persian version of the questionnaire was previously validated by the authors (16).
Patients were randomly assigned to hydrodistention plus cystistat® (intervention) and hydrodistention alone (control) groups using computer-generated random numbers. Patients were instructed not to drink at least 4 h before the procedure, not to use diuretics on procedure days, or to use a diet that exacerbates IC symptoms, such as coffee. First, a urine specimen was collected to check for the absence of infection. If a urinary tract infection was diagnosed, the patient was treated with antibiotics for a week.
Hydrodistention was performed under general anesthesia. First, the urologist performed a cystoscopy and collected urine samples for cytology. The bladder was dilated with normal sterile saline for 1 - 2 min. Then, the bladder was emptied and refilled to allow the urologist to correctly see the lesions and wounds. Therapeutic hydrodistention was continued for another 8 min at 80 - 100 cmH2O pressure. In the intervention group, cystistat® solution (40 mg of sodium hyaluronate in a 50 mL vial) was infused into the bladder through a catheter. Patients had to keep the solution in their bladder for at least 30 minutes, take different positions, and drain it by urinating. This procedure was completed weekly for four weeks and monthly for two months.
In this study, the patients and the doctor knew about the allocated treatment. Nevertheless, the researcher who evaluated the questionnaires and the statistical analyst of the study were completely unaware of each patient's treatment. The data were analyzed using SPSS version 16. The independent t-test was used for variables with normal distribution, and the Mann-Whitney test for variables with non-normal distribution. The chi-square test and Fisher’s exact test were utilized to compare the qualitative variables between the two groups. P-values below 0.05 were considered significant.
All patients signed consent forms before treatment. All stages of this research were conducted following the Declaration of Helsinki (1964) and were approved by the Ethics Committee of Mashhad University of Medical Sciences (registration number: 950419). The study is registered in the Iranian Registry of Clinical Trials, with a primary registry in the WHO Registry Network (registration ID: IRCT20130811014330N5).
4. Results
All our patients were married women. The mean age of the intervention group was 46 ± 9 years, which was not significantly different from the control group (P = 0.71). In addition, the groups were similar in terms of bladder capacity, gravidity, parity, and BMI (Table 1).
| Intervention Group | Control Group | P-Value | |
|---|---|---|---|
| Age (y) | 46.3 ± 9.6 | 44.8 ± 10.2 | 0.71 |
| Gravidity (n) | 3.5 (2 - 9) | 3.5 (2 - 9) | 0.84 |
| Parity (n) | 2.5 (1 - 7) | 3 (1 - 6) | 0.75 |
| BMI (kg/m2) | 23.2 ± 2.9 | 23.8 ± 2.9 | 0.96 |
| Bladder capacity (mL) | 267.0 ± 36.8 | 262.5 ± 38.4 | 0.77 |
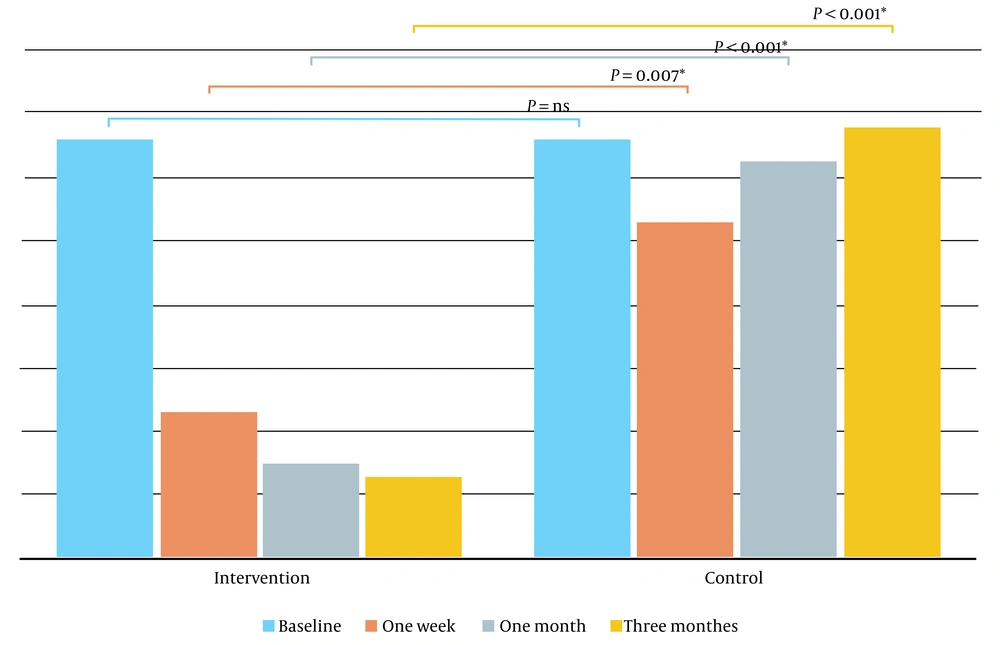
The intervention group had significantly better improvements in overall, one-week, one-month, and three-month measurements, and differences from the control group became more evident over time (Figure 1).
Symptom index score (SI score), the problem index score (PI score), and the subscales of the O`Leary-Sant questionnaire were statistically similar between the two groups at the baseline. In the control group, the mean score of the questionnaire (PI score + SI score) at the baseline was 33.2 ± 1.9. One week post-intervention, only two patients had questionnaire scores less than five, and after three months of treatment, all patients had scores greater than or equal to 30, just like their baseline scores, with no improvements.
In contrast, in the intervention group in which patients received intravesical cystistat in addition to hydrodistention, the mean score of the questionnaire was 32.6 ± 3.6 at the baseline and reached 6.4 ± 12.4 at the end of the third month, which indicates a significantly better enhancement compared to the control group (P < 0.001) (Table 2). In this group, scores of 10 out of 12 patients were below five after three months, indicating symptom relief in 83.3%.
| Baseline | One Week | One Month | Three Months | |||||
|---|---|---|---|---|---|---|---|---|
| SI Score a | PI Score b | SI Score | PI Score | SI Score | PI Score | SI Score | PI Score | |
| Intervention | 17.0 (13 - 20) c | 16.0 (12 - 16) | 4.0 (0 - 20) | 1.0 (0 - 16) | 1.5 (0 - 20) | 0.0 (0 - 16) | 0.5 (0-20) | 0.0 (0 - 16) |
| Control | 17.5 (15 - 20) | 16.0 (14 - 16) | 15.5 (2 - 20) | 15.0 (0 - 16) | 16.5 (9 - 20) | 16.0 (9 - 16) | 18.0 (14 - 20) | 16 .0 (15 - 16) |
| P-value d | 0.97 | 0.93 | 0.05 | 0.01 | 0.001 | 0.001 | < 0.001 | < 0.001 |
a Symptom index score
b Problem index score
c Data are presented as; median (min-max)
d Mann-Whitney test was used for determining P-values
Notably, all questionnaire subscales significantly improved in the intervention group, including urgency, frequency, nocturia, and dysuria. The SI score of nocturia in one week was the only exception, which did not show a significant difference from the control group (P-value = 0.88).
5. Discussion
In this study, disease symptoms resolved in 83.3% of patients who received intravesical instillation of sodium hyaluronate in addition to bladder hydrodistention after three months of treatment, while the patients who only underwent hydrodistention did not show any improvements after three months.
Treatment of IC has always been challenging, and several therapies have been proposed without knowing the exact pathophysiology of the disease. Each of them targets a possible cause of the disease based on empirical observations. The current treatments include oral medications (e.g., Elmiron), intravesical therapy (e.g., dimethyl sulfoxide, heparin, or hyaluronic acid), and even surgical interventions in rare cases (17). However, reports of the efficiency of these medications have been controversial. Nickel et al. recently conducted a double-blind, randomized, placebo-controlled study comparing the efficacy of the currently recommended pentosan polysulfate sodium (Elmiron) dose with one-third of the recommended dose with placebo. In this study, Elmiron showed no therapeutic effect compared to the placebo (18). Intravesical instillation of dimethyl sulfoxide (DMSO) is another treatment. DMSO was reported to be effective in treating a subset of IC/BPS patients (19). However, an unpleasant garlic odor that persists after treatment with DMSO is a problematic side effect. Intravesical heparin has also been reported to be effective in previous studies, and alkalinized lidocaine boosted its efficacy (20, 21). Among all the IC/BPS medications, intravesical hyaluronic acid has shown the best outcomes. Many studies, consistent with our findings, have demonstrated that it has long-term high effectiveness with no significant side effects (22). Shao et al. showed that the effects of hyaluronic acid were superior on heparin (3). A recent systematic review and meta-analysis confirmed the efficacy of intravesical GAG therapy for IC/BPS. They stated that high molecular weight hyaluronic acid (cystistat®) therapy is superior to other instillation regimens (23). According to Raymond et al. (24), intravesical cystistat® can be used for patients with BPS and recurrent UTIs. Moreover, Sommariva et al. studied the effect of intravesical cystistat® on chemical and radiation cystitis, and 97% of patients reported complete relief of dysuria and pain (25). Comparison of our study results with the findings of Welk and Teichman (2008), who treated IC/BPS patients with an intravesical solution of lidocaine, heparin, and sodium bicarbonate shows that intravesical cystistat® has equivalent or even superior effects to this triple intravesical regimen (26).
The limitation of the present study and other similar investigations is the lack of precise criteria for diagnosing and evaluating responses to treatment in IC/BPS patients, making the findings more subjective than objective. This may be problematic when we need to compare the results of different studies. Another limitation, as mentioned before, was that although the researcher who evaluated the questionnaires and the statistical analyst were blinded to different treatment procedures in the two groups, blinding was not possible for the patients and the doctor. The results of this study indicate that using cystistat® solution in treating patients with IC/BPS leads to pain and urinary symptom relief in most patients.
5.1. Conclusions
According to our findings, the intravesical instillation of hyaluronic acid and hydrodistention showed longer-lasting effects compared to hydrodistension alone. However, more research is needed to confirm these results and find the best implementation method.