1. Context
Wilms’ tumor (WT), as the most common renal malignancy in childhood, is responsible for nearly 7% of all childhood cancers (1-4), with an annual incidence rate of about 7 to 10 cases per million in children younger than 15 years of age (5, 6). This disease is not confined to children and can also occur in older children and even adults (7).
Wilms’ Tumor has the ability to extend to the renal pelvis and ureter. The chance of occurrence of ureteric extension is approximately 2% (8). Furthermore, like any other primary malignant renal neoplasm, intravascular extension must be considered in all children with Wilms’ tumor because of its high chance of occurrence. Its incidence was reported 4.1% and 6.0% in a National Wilms tumor study- 3 (NWTS-3) and NWTS-4 trials, respectively (9).
Novel treatment strategies via combining chemotherapy and radiotherapy with total tumor nephrectomy of unilateral WT, has improved the overall survival rate to more than 90% (1, 4, 10-12). Innovative surgical approaches and newer neoadjuvant chemotherapy regimens have minimized treatment-related complications (13). This dramatic advances in the management of Wilms’ tumor have resulted in more than 85% of patients with localized disease being completely cured and 70% surviving a metastatic disease (14). Nowadays, nationally- and internationally-based well-organized coordinated clinical trials and the use of multi-modal therapies have resulted in improved survival (15).
Symptoms: clinical symptoms in favor of having ureteral extension are as follows; flank pain, bloody urine or the presence of tissue or clot in the urine and urethral mass (8, 16, 17).
The most common presentation in WT involving the collecting system is hematuria, which is seen in 87% of patients. This is in contrast with classical WT, in which hematuria is only seen in 25% of cases (18). Preoperative detection of ureteral extension is crucial, since cutting across the tumor in the ureter will spill the tumor, thereby increasing its stage and creating difficulty eliminating the cancer. On the other hand, intra-cardiac tumor thrombus is usually asymptomatic, and this was firstly reported by the NWTS-3 trial (3, 19), and again confirmed by the study of Abdullah et al. (12).
Pathology: adult incidence of WT is less than 1% of all recognized WTs. Although both pediatric and adult Wilms share the histopathological pattern, which consist of 3 parts, blastemal, epithelial and stromal components (20), diagnosing adult Wilms is very challenging. This is due to a number of reasons: 1) Many other adult renal tumors, which are involved in the differential diagnosis, 2) Inadequate specimen for pathologic evaluation, and 3) Lack of systemic work-up, which can lead to misidentification of the tumor (20). Furthermore, from radiological stand point, preoperative diagnosis of adult WT is extremely difficult, which roots from lack of specific radiographic findings that can differentiate it from more common adult renal malignancies (21).
In a review by Kilton et al. (22), six diagnostic criteria for differentiating adult WT, based on pathological and clinical features of the tumor had been introduced, which can still be used today. Pathological features include, 1) blastematous spindle- or round-cell component, 2) tumors with abortive or embryonal tubular epithelial or glomeruloid structure, 3) tumors without any diagnostic areas of renal cell carcinoma, and 4) tumors with illustrated confirmation of pathology. From clinical stand-point, it should be a primary renal neoplasm, found in an adult older than 15 years of age.
Imaging: Diagnosis of ureteral extension in preoperative imaging is not an easy task and the best result in the largest reported series is only 30% (8). The presence of hydronephrosis or nonfunctioning kidney or any expansion of ureter due to soft tissue may increase the suspicion of ureteral involvement. It is worth mentioning that the tumor itself and also the intravascular extension (tumor thrombosis) can mimic the image of nonfunctioning renal unit by exerting external pressure on the ureter (23).
Similar to other adult primary renal neoplasms, the issue of tumor thrombosis also applies to WT. It can extend into the renal vein, inferior vena cava (IVC) and atrium with a prevalence of 11.3%, 4.1% to 8.1%, and 1%, respectively (9, 23, 24). Similar to ureteral extension, preoperative detection of venous tumor thrombosis is crucial. It is necessary to perform preoperative planning for the surgical approach, the need for cardiopulmonary bypass (CPB), and also the decision about neoadjuvant chemotherapy. The infra-hepatic location of the tumor thrombus makes it amenable for the front approach during surgery, while retro-hepatic IVC will necessitate a supra-diaphragmatic IVC control. The supra-diaphragmatic location of the tumor thrombus will mostly require CPB with usually an upfront chemotherapy (23, 25).
Detecting intravascular thrombus and its level was achieved in patients in SIOP 93-01/GPOH and SIOP 2001/GPOH studies, using a combination of modalities. Ultrasound with Doppler was the most commonly used modality (94%), and has been proven to be effective and reliable, and has the benefit of being able to be used intra-operatively if required (15). The second most popular imaging modality used was computed tomography (CT) (76%), which is routinely used in the staging of patients with Wilms, although the sensitivity of identifying tumor thrombus has been lower as is the accuracy of determining the level of thrombus extension (13).
The most recommended imaging modality in pediatric radiology literature for detecting the diameter of IVC and also the presence of flow inside it, is ultrasound with color Doppler evaluation (26-28). Even in cases with unmistakable findings in CT, Doppler ultrasound is a common practice in children with WT.
Magnetic Resonance Imaging (MRI) is also a reasonable option in assessment of vascular patency. It possesses some attractive features including: no harmful radiation, and no need for contrast agent for evaluating vascular anatomy and multi-planar imaging technology. Evaluation of MRI and sonography in regards to accuracy of tumor thrombus detection has shown comparable results in two single institutional studies (29, 30). Like any other modality, MRI has its own drawbacks. It is expensive, not widely available, and takes a long time for capturing images, resulting in the necessity for sedation or even anesthesia. Furthermore, its sensitivity for detecting lung metastases is poor, creating difficulty in staging of WT in children (31).
In a study by Khenna et al. in 2012, the accuracy of current CT imaging technology in detecting tumor thrombus was compared with a sonographic study. In their study, CT could accurately identify the tumor thrombus in 35 out of 38 patients. In those 3 unsuccessful cases, Doppler could only identify one of them. Additionally, CT could predict the thrombus level in 85.1% of already identified patients. The most common reasons that created the limit in CT imaging was anatomical disfigurement, due to a large mass and also large hilar lymph nodes that distorted the hilar vessels (31).
In another part of this study, routine Doppler US was performed in 108 out of 173 patients. This resulted in detecting only 3 additional cases of venous extension that was missed by CT, 2 in the renal vein, and 1 in the IVC at the level of the renal vein. Nevertheless, this additional information would not have changed the surgical plan. Based on these results, they concluded that in cases, in which CT findings are conclusive for tumor thrombus, Doppler imaging is not necessary (31).
The study of Ritchey et al. showed that the use of echocardiography is highly sensitive at identifying intra-atrial extension, demonstrating it in 14 of their 15 patients (9). Hence, it has been suggested that all patients identified as having intravascular extension should undergo echocardiography, as patients with intra-cardiac extension are often asymptomatic (12, 32).
An important reminder is that detailed clinical examination should not be forgotten in the advent of advancing technology. Detecting varicocele can highlight the intravascular extension, as can hepatomegaly and ascites; therefore, a thorough clinical examination is mandatory in all patients with Wilms’ tumor (23).
Staging: according to children’s oncology group (COG) studies (USA) and Societe’ Internationale D’Oncologie Pediatrique (SIOP) classification, ureteric extension is considered as stage 2 disease. Any tumor spill during surgery is classified as stage 3, according to COG. This is to intensify the treatment regimen to lower the chance of recurrence (33).
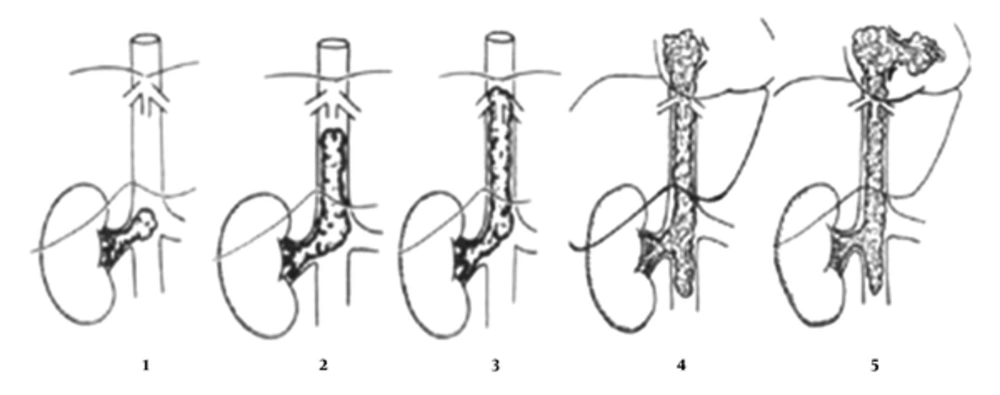
Several staging classifications have been suggested in the past for tumors other than Wilms’ tumor with IVC or atrial extension. In 1982, Cummings suggested a classification system of renal cell carcinoma (RCC) based on the location of the tumor thrombus relative to the diaphragm (34, 35). Subsequently, in 1986, Pritchett et al. redefined classification of thrombus extension in relation to the hepatic vessels in the IVC to three levels; Level I: Infra-hepatic intravascular extension, level II: Intra-hepatic extension, and level III: Supra-hepatic or atrial extension of intravascular thrombus (36).
In 1987, Staehler et al. described a more detailed four-stage system that was modified by Daum in 1994, which considered the extent of intimal attachment of the thrombus (34). He described Stage I as a small < 5 cm thrombus extension in the IVC below the level of the hepatic vessels; Stage II as a large thrombus > 5 cm, yet, still below the hepatic vessels; Stage III as extending to the level of the hepatic vessels and above providing more operative difficulty obtaining proximal control; and Stage IV as tumor thrombus extending to the atrium.
In 2013, Abdullah et al. modified Daum’s classification by adding stage V for intra-ventricular extension of tumor thrombus (Figure 1) (12, 15).
In Stage V, the authors suggested that the respective stage be categorized as a, b or c at the time of surgery to indicate the degree of intimal involvement. Regarding these categories, a indicates a free thrombus, b indicates that the thrombus is adherent to the vessel wall or vessel wall infiltration, and c indicates hepatic vein involvement (which could therefore by definition only be added to stages III to V) (12) (Table 1).
| Stage | Description |
|---|---|
| Ia | Small (< 5 cm) infra-hepatic IVC tumor thrombus |
| Ib | Subintimal attachment of small (< 5cm) infra-hepatic IVC tumor thrombus |
| IIa,b | Large thrombus (> 5cm) below the level of hepatic veins |
| IIIa,b,c | Thrombus extending to the hepatic vein junction |
| IVa,b,c | Thrombus extending to the right atrium |
| Va,b,c | Thrombus extending into the right ventricle |
Intra-Caval Thrombus Staging Classification as Modified by Abdullah et al. (12)
Treatment: Due to rarity of WT in adults and lack of established treatment guidelines, it is treated according to accepted protocols in children. Previously, there was general consensus that because of its poor prognosis, all stages of adult WT should be treated by an aggressive multi-stage protocol, including surgery, chemotherapy, and radiotherapy (35). However, based on the national Wilms’ tumor study group trials, they recommended that all adult WT patients with favorable histology should be treated like the children’s protocol, according to stage (37).
The NWTSG and SIOP investigators have different views regarding the treatment protocol. The NWTSG has always favored upfront nephrectomy, while SIOP supported the notion of pre-nephrectomy chemotherapy in all patients older than 6 months of age to ease the surgery by reducing size and prohibiting spillage during operation (14, 38, 39).
Recently, the United Kingdom children’s cancer study group (UKCCSG) completed a randomized trial regarding comparison of the two aforementioned approaches. They concluded that neoadjuvant chemotherapy creates favorable results regarding stage distribution and surgical complications and also causes significant reduction in the overall treatment, which the patient should receive. Despite differences in each approach, both treatment strategies, by UKCCSG and SIOP, show almost equivalent outcomes and trials still investigate the benefits of each approach (40, 41).
According to the NWTS-5 study, WT in a solitary or in horseshoe kidney, supra-hepatic IVC tumor thrombus, and in children with respiratory distress due to extensive pulmonary metastases, pre-operative chemotherapy, is recommended (42). However, SIOP favors primary chemotherapy in all patients even stage I, except in children younger than 6 months of age (14). Regarding overall and relapse-free survival, both strategies are similar (41, 43). This is why current studies are mostly focusing on strategies to lower treatment-related complications and morbidities (44).
Chemotherapy has an undeniable role in treating WT (11, 32, 45). It reduces the size of the tumor and eases the surgical removal with lower morbidity (3, 13, 24, 46). Even complete dissolution of tumor thrombus is reported (10, 47). In a series by Abdullah et al. (12) complete regression of atrial tumor thrombus by chemotherapy obviated the need for CPB in 2 patients. In these 2 patients, nephrectomy and tumor thrombectomy were performed by controlling infra- and supra-hepatic IVC in addition to occlusion of liver veins (Pringle maneuver). Nevertheless, progressive intra-atrial tumor growth, with the risk of tumor pulmonary embolus, or any influence on cardiac function are indications for urgent surgery and no time should be wasted for chemotherapy (12).
It is worth mentioning that preoperative chemotherapy has its own risks. In a report by Shamberger et al. 5 out of 69 patients receiving neoadjuvant chemotherapy had complications, among which one ended up in fatality due to acute respiratory distress syndrome (ARDS). Others were tumor embolus in 1, tumor progression in 1, and ARDS in 2 cases (20). Therefore, neoadjuvant chemotherapy requires close surveillance for evidence of tumor regression and possible complications. Failure of regression may mandate early resection. Based on tumor response to chemotherapy with regards to the extent of thrombus, volume, appearance of the thrombus, and the presence of atrial extension on imaging, elective surgical resection should be planned (48). Chemotherapy should be continued until the tumor is considered resectable by the treating surgical team. This time varies in different studies; Shamberger et al. found a median treatment duration of 8 weeks, Szavay et al. described a 4-week duration, while Murthi et al. prescribed neoadjuvant chemotherapy for up to 29 weeks (13, 23, 45).
In a nutshell, indications for primary resection are as follows (15):
1) Unstable tumor thrombus on a narrow pedicle identified on echo (Murthi et al.) (45)
2) Traumatic tumor rupture (Szavay et al.) (13)
3) Child with Budd Chiari syndrome (Abdullah and Mushtaq) (12, 31)
4) No tumor response or even tumor progression during chemotherapy (Ritchel et al.) (47)
5) Intra-ventricular tumor growth with the risk of pulmonary embolus or any influence on cardiac function (Abdullah et al.) (12)
The main disadvantage that has been suggested for pre-operative chemotherapy is that it will compromise the staging of the tumor and cause alterations in histological information (13).
Aside from causing tumor shrinkage, neoadjuvant chemotherapy may facilitate resection by creating accessibility to tissue planes. Tumor size reduction by at least 50% has been reported in the literature (13). Additionally, chemotherapy has the advantage of eradicating organ metastases, especially in the lungs. In Szavay’s report, in 6 out of 16 children, who had lung metastasis, chemotherapy alone was used as the treatment strategy (13).
Surgery in these patients is very challenging and requires a planned approach with well described and established techniques (48-50). An experienced cardiovascular surgeon should be available and the procedure should be done in an operation room with cardiopulmonary bypass capabilities. Cardiopulmonary Bypass with hypothermia is a safe procedure and is recommended when extensive intravascular tumor resection is anticipated (2, 3, 25, 51, 52). Diverting blood out of the surgical field during CPB creates an excellent situation regarding surgical site view and IVC reconstruction after thrombectomy. In addition, it prevents tumor embolization, which could be catastrophic (3, 25). Hypothermia creates the opportunity of a longer operation time if aorta is clamped during surgery because of better tolerance of internal organs, such as the liver and kidneys, to cold ischemia (13).
Inevitably, adopting CPB, will prolong the operation time and will necessitate post-op anticoagulation. However, major complications are not so common. In a report by Szavay et al. (13), about the results of tumor thrombectomy under CPB in 9 patients, no intra-operative deaths were encountered. One case was complicated by major hemorrhage, in which the operation was performed under an emergency situation. Survival of patients was good except for the child with unresectable primary tumor due to local tissue infiltration.
Complications of surgery: Morbidity from surgery should always be kept in mind. In NWTS-3, the occurrence rate of surgical complications after primary nephrectomy for Wilms’ tumor was 19.8% (53). The most common ones were hemorrhage and small-intestinal obstruction. Unfavorable tumor characteristics, eg, advanced local tumor stage and intravascular extension, or erroneous surgical plan and en bloc resection of other visceral organs are the known risk factors. Thus, preoperative chemotherapy in certain at risk patients was recommended (19, 54). These high-risk groups included patients with supra-hepatic IVC extension and also those patients, who had large inoperable tumors. En bloc resection of adjacent organs in large WT is not advocated because it mostly compresses the neighboring organs instead of invading them.
These risk factors were also confirmed by the NWTS-4 report (47). Advanced local tumor stage, tumor size larger than 10 cm, IVC or intra-atrium extension, an erroneous surgical technique, and inexperienced surgeon are all associated with an increased risk of surgical complications.
In the SIOP study, the incidence of surgical complications was reported as 8%. As mentioned earlier, the SIOP group advocated preoperative chemotherapy, which can significantly shrink the tumor size, facilitating its surgical excision. In their report, unlike the NWTSG study, they also included tumor ruptures during surgery as a complication, thus the incidence of intra-operative and post-operative nephrectomy-related surgical complications was lower than 8%. Occurrence of small-bowel obstruction, which was 5% in the NWTS-4 review, was 2.5% in the SIOP report (55, 56).
Outcome: Ureteral extension of Wilms’ will not compromise the clinical outcome and patients have an excellent prognosis with expected long-term survival of > 90%, provided that tumor resection is complete. In these children with complete resection, adjuvant radiation therapy or 3-drug chemotherapy is not necessary (8).
Neither the presence of intravascular disease nor its extent appears to influence the long-term outcome of patients with intravascular tumor thrombosis (9). In a report of 165 patients with intravascular extension compared to 1621 patients without intravascular extension by Shamberger et al. (20), they found no adverse effect on the 3-year relapse-free survival rate, considering the stage or pathologic subgroup. This observation was again proved in the review by Murthi et al. (45), in which recurrence in the intravascular compartment was not responsible for relapse and death of patients. Furthermore, according to Murthi’s report, the presence of viable tumor cells in the excised tumor thrombus, despite preoperative chemotherapy, will not deteriorate the prognosis with increasing local recurrence in vascular compartment.
Interestingly, incomplete removal of tumor thrombus does not necessarily end up in vascular recurrences, as Shamberger et al. (23) have reported no increased incidence of relapse in their 18 patients with incomplete surgery. This denotes that ‘gross clearance’ is somehow preferable to extensive clearance of caval and atrial lumen of all tumor thrombus at any cost (45).
In a nutshell, neither the level of tumor thrombus nor the use of CPB was predictive of survival (13), although, intravascular extension may increase the incidence of surgical complications (3, 9) with an odds ratio of 2.2. Most papers denote that the single best predictor of survival is the histological subtype (13, 19, 23, 57). There is no association between unfavorable histology and propensity to have intravascular tumor thrombus (23).
Adult WT prognosis is significantly worse compared to children (58). This may be due to advanced stage of cancer upon diagnosis, however, even at a similar stage, adult WT still appears worse compared to children (36).
2. Conclusion
Ureteral extension of WT is an unusual phenomenon. The surgeon should be vigilant about possible clues of intraluminal extension e.g. gross hematuria or the presence of hydronephrosis. Once identified, it should be completely resected. This may necessitate resection of the bladder cuff to obtain negative surgical margin for tumors extending out of the ureteral orifice. If completely resected, it will result in excellent prognosis.
Intravascular and intracardiac extension must be excluded in all patients with Wilms’ tumor. With accurate identification of this problem, subsequent preoperative chemotherapy and resection with an appropriately skilled team, the survival rates are not significantly different from those with uncomplicated Wilms’ tumor.