1. Background
The best method to assess renal function is measuring the glomerular filtration rate (GFR) (1). Chronic kidney disease (CKD) patients are classified into stages 1-5 based on GFR. Inulin has been used as a gold standard indicator for measuring GFR (2). Other substances used as filtration markers include 125 Iothalamate, chromium-51 ethylenediaminetetraacetic acid, and Iohexol (3). However, these substances are not often used because they are time-consuming, expensive, and difficult to process (4). Ideally, GFR should be determined with an accurate, inexpensive method. Estimation of GFR can be done using Modification of Diet in Renal Disease (MDRD), abbreviated MDRD, or Cockcroft and Gault (C-G) equations (5), which can present different results. Differences in results are due to the standardization of analytical techniques, the rate of production and tubular secretion of creatinine (6).
Furthermore, creatinine concentration is not only affected by GFR but also affected by other factors such as diet, muscle mass, gender, and age.
The best marker to estimate GFR should be a product that is produced continuously in the body and excreted by the kidney via free glomerular filtration without tubular absorption or secretion (7).
Cystatin C has been proposed as a new filtration marker to substitute creatinine, and it is very sensitive to changes in GFR (8). Cystatin C is a low molecular-weight basic protein (13 kDa) that is produced at a constant rate by all nucleated cells of the body and freely filtered by the glomeruli of the kidney without tubular secretion and absorption (8).
Some investigators have suggested that serum cystatin C has advantages over serum creatinine to estimate GFR as it is not affected by age, gender, muscle mass, drugs, and diet (9, 10). However, some other investigators have questioned this idea (11). To determine GFR based on serum cystatin C, several equations such as Larsson, Hoek, Filler, Le Bricon, and Rule have been proposed (12-16).
Studies have shown that BMI is related to the serum levels of cystatin C (17-19). The mechanism of this association is unclear, but experimental evidence suggests that adipocyte tissues secrete large amounts of cystatin C, which can lead to the increased levels of serum cystatin C in obese individuals with high BMI (20). According to this assumption, estimated GFRs based on cystatin C formulas in obese cases may be different from those estimated by the gold standard. Whereas, in high BMI cases, it is expected that the result of GFR estimation by creatinine-based formulas be different from that of cystatin C-based equations.
In this study, we compared cystatin C-based equations with creatinine based formulas to identify the most precise formula to estimate GFR in Iranian population diagnosed with CKD. Also, the relation of these formulas results with patients BMI, age, and sex is presented.
2. Methods
In this cross-sectional study, we investigated 120 patients with CKD, whose 3-month baseline GFR was less than 60 mL/min per 1.73 m2.
Our exclusion criteria were pregnancy, liver cirrhosis, hypothyroidism, hyperthyroidism, or administration of any medication interfering with creatinine tubular secretion. Each subject gave his/her informed consent.
Non-fasting morning blood samples were taken and analyzed for serum creatinine, urea, and cystatin C. The Jaffe method was used to measure creatinine with a Boehringer Mannheim/Hitachi 747 analyzer. Serum cystatin C levels were determined with the Human cystatin C ELISA kit using Bio Vendor Laboratory Medicine, AS. The calibrated creatinine was used in all analyses involving the MDRD equations. GFR was estimated using the creatinine-based MDRD, Abbreviated MDRD, and C-G equations (21) as well as the cystatin C-based Le Bricon (12), Filler (13), Hoek (14), Larsson (15), and Rule (16) equations per surface area of 1.73 m2, as shown in Table 1.
| Equation | Formula, mL/min/1.73 m2 |
|---|---|
| Cystatin C-based | |
| Filler | Log (GFR) = 1.962 + (1.123 × log (1/cystatin C)) |
| Le Bricon | GFR = (78 × (1/cystatin C)) + 4 |
| Hoek | GFR = -4.32 + (80.35 × 1/cystatin C) |
| Larsson | GFR = 77.24 × cystatin C-1.2623 |
| Rule | GFR = 76.6 × cystatin C-1.16 |
| Creatinine-based | |
| MDRD | GFR = 170 × creatinine-0.999 × age-0.176 × blood urea nitrogen-0.170 × albumin0.318 × (0.762 for women) ×(1.180 for African-Americans) |
| Abbreviated MDRD | GFR = 186 × creatinine-1.154 × age-0.203 × (0.742 for women) × (1.212 for African-Americans) |
| Cockcroft and Gault | GFR = ((140 - age) × body weight)/(creatinine × 72) × (0.85 for women) |
For analysis, the patients were divided into three categories according to their BMI as: normal weight (BMI: 18.5 to < 25), overweight (BMI: 25 - 30), and obese (BMI > 30). They were also divided into three groups according to their age as: under 40 years, 40 to 60 years, and more than 60 years.
2.1. Statistical Analyses
SPSS software (version 20.0) was used to analyze data. The normality of the distribution of variables was checked by one- sample Kolmogorov-Smirnov test. Pearson correlation coefficient was used to examine relations among quantitative variables. Linear regression models were used to determine relations among different methods of GFR assessment. Chi-square was used to compare qualitative variables in groups, two sample t-test to compare quantitative variables between two groups, and one-way analysis of variance (ANOVA) to compare quantitative variables between multiple groups. Student’s t-test was used to evaluate each parameter in female and male groups. Spearman rank correlation, Mann Whitney U test, and Kruskal Wallis test were used for variables with non-normal distribution as appropriate non-parametric tests. A P value less than 0.05 was considered significant.
3. Results
Out of 120 patients, 45.8% (55) were female and 54.2% were male (65). The mean age of patients was 56.4 ± 16 years. The patients’ height ranged from 143 to 180 cm and the mean height was 163 ± 8 cm. The mean weight of patients was 68.2 ± 11 kg ranging from 37 to 95 kg. The mean BMI of the patients was 25.6 ± 3 kg/m2, mean creatinine was 2.2 ± 0.6 mg/dL, and mean cystatin C was 1.7 ± 0.5 mg/L.
The other characteristics of patients are shown in Table 2.
| Characteristics | Value |
|---|---|
| Male | 55 (45.8) |
| Female | 65 (54.2) |
| Age, y | 56.41 ± 16.4 |
| Hight, cm | 163.04 ± 8.4 |
| Weight, Kg | 68.22 ± 11.7 |
| BMI, kg/m2 | 25.65 ± 3.9 |
| Serum creatinine, mg/dL | 2.20 ± 0.65 |
| Serum cystatin C, mg/L | 1.73 ± 0.51 |
| Serum Albumin, g/dL | 4.55 ± 0.47 |
| BUN, mg/dL | 41.76 ± 18.3 |
aValues presented as number (percentage) for sex and mean ± SD (range) for other variables.
The mean values of estimated GFRs by three creatinine-based formulas (C-G, MDRD, and abbreviated MDRD) and five cystatin C-based equations (Filler, Le Bricon, Larsson, Rule, and Hoek) are demonstrated in Table 3. Also, the frequencies of different CKD stages according to the GFR estimating formulas are shown in Table 3.
| Variables | Value | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 |
|---|---|---|---|---|---|---|
| Cockcroft and Gault | 36.26 ± 14.33 | 0 | 7 (5.8) | 72 (60) | 36 (30) | 5 (4.2) |
| Abbreviated MDRD | 32.44 ± 11.38 | 0 | 1 (0.8) | 62 (51.7) | 53 (44.2) | 4 (3.3) |
| MDRD | 32.69 ± 11.44 | 0 | 2 (1.7) | 63 (52.5) | 52 (43.3) | 3 (2.5) |
| Rule | 45.12 ± 17.27 | 3 (2.5) | 12 (10) | 91 (75.8) | 14 (11.7) | 0 |
| Larsson | 43.69 ± 18.52 | 3 (2.5) | 9 (7.5) | 91 (75.8) | 17 (14.2) | 0 |
| Hoek | 46.22 ± 16.26 | 3 (2.5) | 13 (10.8) | 90 (75) | 14 (11.7) | 0 |
| Le Bricon | 53.06 ± 15.78 | 4 (3.3) | 19 (15.8) | 92 (76.7) | 5 (4.2) | 0 |
| Filler | 54.78 ± 20.18 | 7 (5.8) | 21 (17.5) | 83 (69.2) | 9 (7.5) | 0 |
aValues presented as number (percentage) for sex and mean ± SD (range) for other variables; GFRs expressed as mL/min/1.73 m2.
3.1. Correlation Between Creatinine-Based and Cystatin C-Based Equations for GFR Estimation
There was a strong significant relationship among GFR values estimated by three creatinine-based formulas (r: 0.74 to 0.98, P value < 0.0001). Also, the correlation coefficient showed that there are significant correlations among all the five cystatin C-based GFRs estimating equations (r: 0.99 to 1, P value < 0.0001).
In addition, based on the results, all of the creatinine-based and cystatin C-based equations had a significant correlation for GFR estimation (P value < 0.001), as demonstrated in Table 4.
| Cystatin C-Based Equations | Creatinine-Based Equations | |||||
|---|---|---|---|---|---|---|
| Cockcroft and Gault | Abbreviated MDRD | MDRD | ||||
| r | P value | r | P value | r | P value | |
| Rule | 0.384 | < 0.001 | 0.400 | < 0.001 | 0.383 | < 0.001 |
| Larsson | 0.380 | < 0.001 | 0.396 | < 0.001 | 0.379 | < 0.001 |
| Hoek | 0.391 | < 0.001 | 0.407 | < 0.001 | 0.390 | < 0.001 |
| Le Bricon | 0.391 | < 0.001 | 0.407 | < 0.001 | 0.390 | < 0.001 |
| Filler | 0.386 | < 0.001 | 0.402 | < 0.001 | 0.375 | < 0.001 |
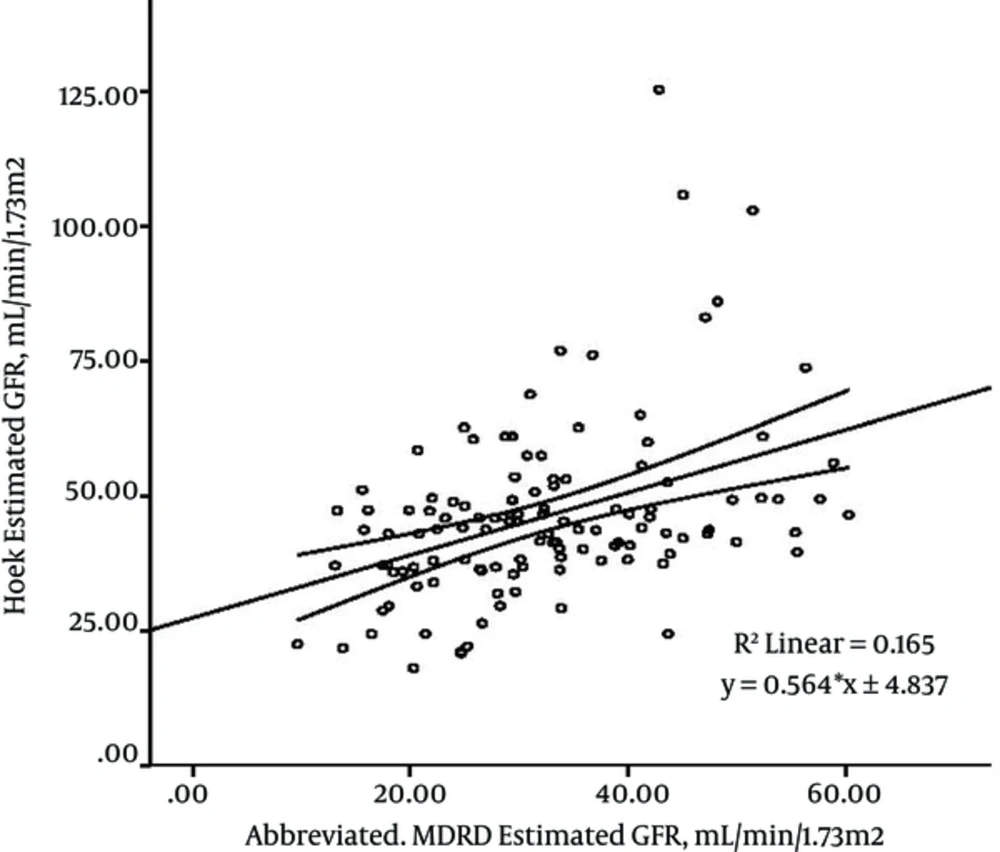
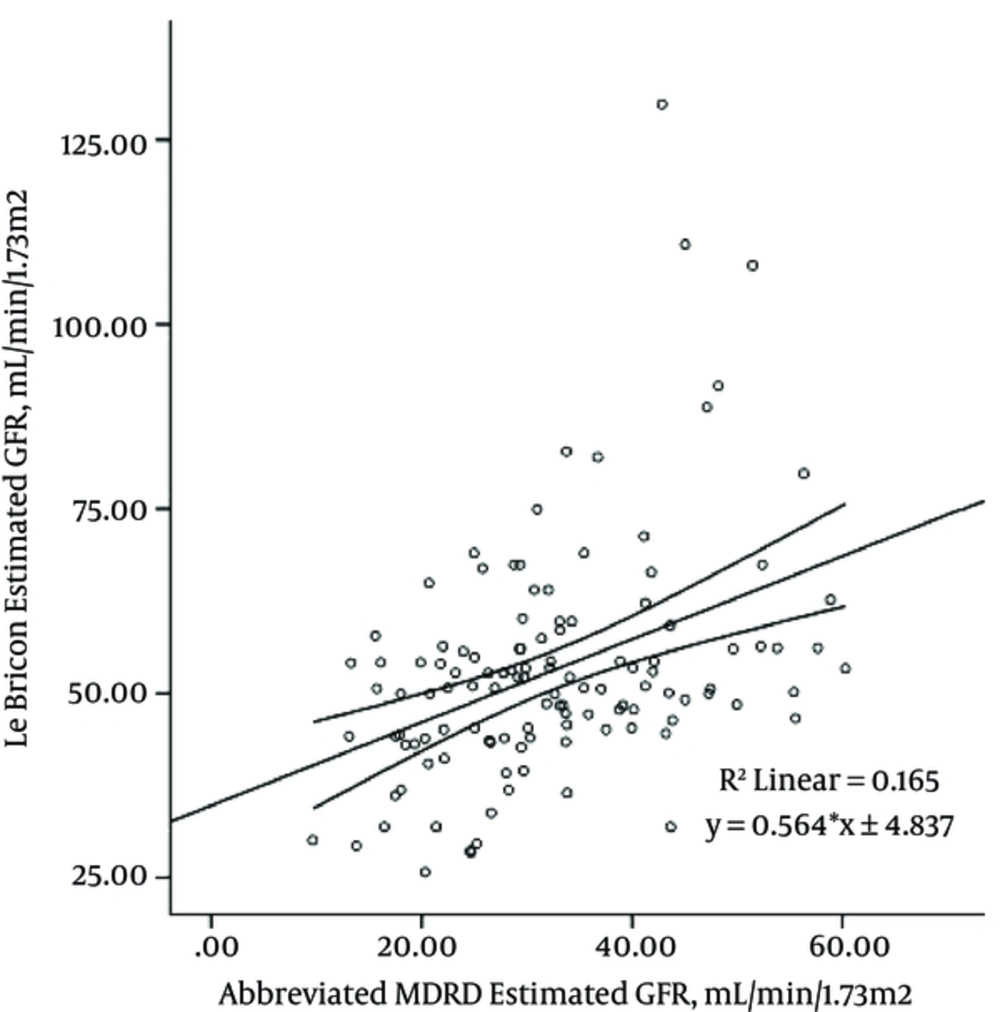
The linear regression model of creatinine-based formulas and cystatin C-based equations for GFR estimation indicated that the most significant correlations were between abbreviated MDRD formula and Hoek and Le Bricon equations (r: 0.40), as shown in Figure 1 and 2.
As shown in Figure 1 and 2, the estimated GFRs by abbreviated MDRD are able to predict 16% of variances of the Hoek-estimated GFRs and Le Bricon-estimated GFRs (R square: 0.165).
The result of the study showed that there is a significant correlation between serum cystatin C and serum creatinine (r: 0.48, P value < 0.001). On the other hand, there was not any significant correlation between BMI and creatinine (r: -0.01, P value: 0.91,) and cystatin C (r: -0.009, P value: 0.92).
3.2. Association Between Creatinine-Based and Cystatin C-Based Equations for GFR Estimation and Their Relationship with BMI, Age, and Sex
The correlation between BMI and GFRs estimated by creatinine based-formulas is demonstrated in Table 5. Mean GFR estimated by C-G equation was higher in the cases with higher amount of BMI (P value < 0.001). However, changes in BMI did not show any significant relationship with the mean GFR estimated by other formulas (P value: 0.9).
| Variables | Body Mass Index | Age, y | Gender | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Weight | Over weight | Obesity | P Value | < 40 | 40 - 60 | > 60 | P Value | Male | Female | P Value | |
| Cockcroftand Gault | 30.5 ± 11.1 | 37.9 ± 13.3 | 47.1 ± 18.0 | < 0.001 | 43.2 ± 15.2 | 41.9 ± 14.9 | 29.5 ± 10.1 | < 0.001 | 31.9 ± 11.7 | 39.9 ± 15.3 | 0.003 |
| Abbreviated MDRD | 33.3 ± 12.2 | 32.1 ± 10.4 | 33.5 ± 12.2 | 0.8 | 35.7 ± 13.3 | 32.4 ± 11.7 | 31.2 ± 10.2 | 0.6 | 26.6 ± 8.8 | 37.3 ± 11.1 | < 0.001 |
| MDRD | 32.4 ± 12.2 | 32.8 ± 10.6 | 33.0 ± 11.9 | 0.9 | 36.5 ± 13.8 | 32.1 ± 11.7 | 31.6 ± 10.0 | 0.5 | 27.2 ± 9.1 | 37.3 ± 11.2 | < 0.001 |
| Rule | 44.8 ± 18.9 | 45.1 ± 13.7 | 45.8 ± 22.2 | 0.9 | 44.0 ± 9.6 | 49.4 ± 22.7 | 42.1 ± 13.6 | 0.4 | 45.0 ± 17.6 | 45.2 ± 17.1 | 0.8 |
| Larsson | 43.4 ± 20.3 | 43.6 ± 14.6 | 44.6 ± 23.7 | 0.9 | 42.4.2 ± 10.0 | 48.3 ± 24.7 | 40.5 ± 14.2 | 0.4 | 43.6 ± 19.0 | 43.8 ± 18.2 | 0.8 |
| Hoek | 45.8 ± 17.8 | 46.4 ± 12.9 | 46.7 ± 20.9 | 0.9 | 45.4 ± 9.3 | 50.2 ± 21.0 | 43.4 ± 13.3 | 0.4 | 46.1 ± 16.4 | 46.3 ± 16.2 | 0.8 |
| Le Bricon | 52.6 ± 17.3 | 53.2 ± 12.5 | 53.7 ± 20.3 | 0.9 | 52.3 ± 9.1 | 56.9 ± 20.4 | 50.3 ± 12.9 | 0.4 | 52.9 ± 15.9 | 53.1 ± 15.7 | 0.8 |
| Filler | 54.3 ± 22.1 | 54.8 ± 16.0 | 55.6 ± 25.9 | 0.9 | 53.6 ± 11.3 | 59.7 ± 26.5 | 51.2 ± 16.0 | 0.4 | 54.6 ± 20.5 | 54.8 ± 19.9 | 0.8 |
The results showed that increases in age significantly reduce the mean GFR generated by C-G formula (P value < 0.0001). However, changes in age did not show any significant correlation with the mean GFR estimated by other formulas.
A relation was detected between GFRs from creatinine-based formulas and gender, but such a relation was not found for GFRs from cystatin C-based equations.
3.3. The Prevalence of Stages 3 - 4 CKD by Using Creatinine-Based and Cystatin C-Based Formulas, and Their Relation with BMI, Age, and Sex
As presented in Table 6, the prevalence of stages 3-4 CKD is significantly lower in patients with higher BMI than patients with lower BMI (P value: 0.005) by using C-G formula, while no appreciable difference was seen between BMI groups using other formulas. In addition, the prevalence of stages 3 - 4 CKD, estimated by C-G and MDRD formulas, was affected significantly by age (P value: 0.03 and 0.008, respectively). Also, there was no significant sex difference in the prevalence of stages 3 - 4 CKD.
| Variables | Body Mass Index, kg/m2 | Age, y | Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Weight (18.5 - 24.9) | Over weight (25.0 - 29.9) | Obesity (≥ 30.0) | P value | < 40 | 40 - 60 | > 60 | P value | Female | Male | P value | |
| No. | 50 | 52 | 18 | 21 | 44 | 55 | 55 | 65 | |||
| Cockcroft and Gault | 49 (98) | 50 (96.2) | 14 (77.8) | 0.005 | 18 (85.7) | 40 (90.9) | 55 (100) | 0.030 | 54 (98.2) | 59 (90.8) | 0.12 |
| Abbreviated MDRD | 49 (98) | 52 (100) | 18 (100) | 0.49 | 20 (95.2) | 44 (100) | 55 (100) | 0.093 | 55 (100) | 64 (98.5) | 1.0 |
| MDRD | 48 (96) | 52 (100) | 18 (100) | 0.24 | 19 (90.5) | 44 (100) | 55 (100) | 0.008 | 55 (100) | 63 (96.9) | 1.0 |
| Rule | 44 (88) | 45 (86.5) | 16 (88.9) | 0.95 | 20 (95.2) | 36 (81.8) | 49 (89.1) | 0.27 | 50 (90.9) | 55 (84.6) | 0.41 |
| Larsson | 45 (90) | 47 (90.4) | 16 (88.9) | 0.98 | 20 (95.2) | 37 (84.1) | 51 (92.7) | 0.24 | 50 (90.9) | 58 (89.2) | 0.49 |
| Hoek | 43 (86) | 45 (86.5) | 16 (88.9) | 0.95 | 19 (90.5) | 36 (81.8) | 49 (89.1) | 0.48 | 49 (89.1) | 55 (84.6) | 0.83 |
| Le Bricon | 39 (78) | 44 (84.6) | 14 (77.8) | 0.65 | 16 (76.2) | 33 (75) | 48 (87.3) | 0.25 | 46 (83.6) | 51 (78.5) | 0.49 |
| Filler | 39 (78) | 41 (78.8) | 12 (66.7) | 0.55 | 16 (76.2) | 31 (70.5) | 45 (81.8) | 0.41 | 43 (78.2) | 49 (75.4) | 0.83 |
aValues are expressed as No. (%).
3.4. Difference Between Creatinine-Based and Cystatin C-Based Formulas for GFR Estimation and Their Correlation with BMI, Age, and Sex
The differences between GFRs estimated by cystatin C-based equations and those estimated by C-G equation are lower at higher BMI levels (P value: 0.004 - 0.01), as shown in Table 7.
| Variables | Body Mass Index, kg/m2 | AGE, y | Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Differences between cystatin-based and creatinine-based equations | Normal Weight (18.5 - 24.9) | Over weight (25.0 - 29.9) | Obesity (≥ 30.0) | P Value | < 40 | 40 - 60 | > 60 | P Value | Female | Male | P Value |
| Rule-Cockcroft-Gault | 14.20 | 7.23 | -1.20 | 0.004 | 0.79 | 8.09 | 12.58 | 0.031 | 13.11 | 5.3 | 0.015 |
| Larsson-Cockcroft-Gault | 12.8 | 5.70 | -2.24 | 0.007 | 0.86 | 7.01 | 10.94 | 0.045 | 11.6 | 3.8 | 0.021 |
| Hoek-Cockcroft-Gault | 22.09 | 15.30 | 6.47 | 0.002 | 9.02 | 15.58 | 20.76 | 0.018 | 21.06 | 13.2 | 0.01 |
| Le Bricon-Cockcroft-Gault | 22.09 | 15.30 | 6.47 | 0.002 | 9.02 | 15.58 | 20.76 | 0.018 | 21.06 | 13.2 | 0.01 |
| Filler-Cockcroft-Gault | 23.79 | 16.94 | 8.52 | 0.01 | 10.32 | 18.45 | 21.73 | 0.078 | 22.7 | 14.9 | 0.03 |
| Rule-abbreviated MDRD | 12.44 | 13.02 | 12.32 | 0.98 | 8.31 | 17.02 | 10.87 | 0.073 | 18.3 | 7.8 | <0.001 |
| Larsson-abbreviated MDRD | 11.04 | 11.49 | 11.08 | 0.99 | 6.65 | 15.95 | 9.23 | 0.068 | 16.9 | 6.4 | 0.001 |
| Hoek-abbreviated MDRD | 13.48 | 14.26 | 13.17 | 0.95 | 9.67 | 17.79 | 12.12 | 0.082 | 19.4 | 8.9 | <0.001 |
| Le Bricon-abbreviated MDRD | 20.33 | 21.10 | 20.00 | 0.95 | 16.54 | 24.52 | 19.05 | 0.083 | 26.3 | 15.8 | <0.001 |
| Filler-abbreviated MDRD | 22.03 | 22.73 | 22.06 | 0.98 | 17.85 | 27.38 | 20.02 | 0.073 | 28.0 | 17.5 | 0.002 |
| Rule-MDRD | 12.38 | 12.33 | 12.83 | 0.99 | 7.46 | 17.28 | 10.44 | 0.40 | 17.7 | 7.9 | 0.001 |
| Larsson-MDRD | 10.98 | 10.80 | 11.59 | 0.98 | 5.81 | 16.20 | 8.80 | 0.038 | 16.3 | 6.4 | 0.002 |
| Hoek-MDRD | 13.41 | 13.57 | 13.68 | 0.99 | 8.83 | 18.05 | 11.69 | 0.044 | 18.9 | 8.9 | <0.001 |
| Le Bricon-MDRD | 20.27 | 20.41 | 20.50 | 0.99 | 15.69 | 24.77 | 18.62 | 0.044 | 25.7 | 15.8 | <0.001 |
| Filler-MDRD | 21.96 | 22.04 | 22.56 | 0.99 | 17.00 | 27.64 | 19.59 | 0.043 | 27.4 | 17.5 | 0.004 |
The differences between GFRs estimated by cystatin C-based equations and those estimated by C-G and MDRD equations were greater in older patients (P value: 0.01 - 0.04).
The differences between GFRs estimated by cystatin C-based equations and creatinine-based equations were significantly different between males and females (P value: 0.15 - 0.001).
4. Discussion
Chronic kidney disease is an important public health problem. Estimation of GFR is essential for the evaluation of kidney function in CKD patients. The current kidney disease outcomes quality initiative (K/DOQI) guidelines classify CKD into five stages based on GFR estimations (10).
There is evidence showing that using creatinine concentration as an ideal marker for GFR estimation is accompanied by problems (22).
Studies have reported that cystatin C is less affected by age, gender, and muscle mass when compared to serum creatinine (23) and it is not affected also by dietary protein intake, inflammation, fever, and agents such as proteins and bilirubin (24).
Our aim in this study was to compare cystatin C-based equations with creatinine based-formulas for estimation of GFRs.
Many studied have suggested that cystatin C concentration is a better indicator to estimate GFR than serum creatinine concentration (9, 10, 25-30), especially in patients with spinal injury, liver cirrhosis, cystic fibrosis, diabetes, and old age (31). While, other investigators have reported that these two substances are equally accurate (14, 32-35).
Also, some researchers believe that many factors such as male sex, weight, height, cigarette smoking, higher serum C reactive protein levels, steroid therapy, and rheumatoid arthritis can increase the serum level of cystatin C. Thus, this substance may be less accurate to evaluate kidney function (35-38).
In this study, we calculated the GFRs of 120 patients with CKD based on creatinine and cystatin C equations. The results indicated that there was a significant correlation between all creatinine-based estimated GFRs and cystatin C-based estimated GFRs (P value < 0.001), and abbreviated MDRD-estimated GFRs were comparable with those of Hoek and Le Bricon equations in CKD subjects (P value < 0.001, r: 0.4, R2: 0.16).
In general, only the mean estimated GFR resulted from C-G formula was under the influence of BMI (P value < 0.001), because it was higher in individuals with higher BMI. Increasing age significantly reduced the mean estimated GFR (P value < 0.0001). This result was expected due to the influence of age and weight on C-G formula. But, we do not have any explanation why BMI and age were not effective in other creatinine-based equations (Table 5).
In addition, a relationship was verified between GFRs estimated by creatinine-based formulas and gender, but such a relation was not found in GFRs from cystatin C-based equations. Since the factors of weight, age, and gender are not considered in the cystatin C-based formulas, this result is not surprising.
On the other hand, since the number of patients on stages 2 and 5 CKD was low, we evaluated the effect of BMI, age, and sex on the prevalence of stages 3 - 4 CKD. The prevalence of stages 3 - 4 CKD, determined by C-G and MDRD formulas, was affected significantly by age (P value: 0.03 and 0.008, respectively).
Also, BMI had an influence on the prevalence of stages 3 - 4 CKD in creatinine-based formulas although it was not statistically significant. The effect of BMI was statistically significant only when C-G formula was used (P value: 0.005). Patients with higher BMI had lower frequency in stages 3 - 4 CKD.
Also, there were no significant sex differences in the prevalence of stages 3 - 4 CKD patients (P value > 0.05) by using creatinine-based formulas.
Differences in estimated GFRs between cystatin C-based equations and C-G and MDRD equations were greater in older patients (P value: 0.01 - 0.04, F: 3.2 - 4.1).
In terms of differences between GFRs estimated by cystatin C-based equations and GFRs estimated by creatinine-based formulas, they were lower at higher BMI levels when C-G equation was used (P value: 0.004 - 0.01), and they were greater in older patients when C-G and MDRD equations were used (P value: 0.01 - 0.04). The differences were also significant between males and females (P value: 0.15 - 0.001).
According to sex, all the creatinine-based formulas showed significant differences while cystatin C-based formulas did not. This result was predictable due to the influence of sex factor in creatinine-based equations, while sex factor is not considered in cystatin C-based equations.
4.1. To Summarize
Our findings revealed that the abbreviated MDRD formula, among creatinine-based GFR formulas, provides GFRs closer to GFRs estimated by cystatin C-based equations in CKD patients. Since the cost of laboratory tests for cystatin C is higher and it does not show superiority over MDRD, we recommend that the abbreviated MDRD formula be used for estimating GFR in CKD patients.
4.2. Limitation
There are two limitations for this study: 1) lack of a gold standard test and, 2) the small number of patients in the study.