1. Introduction
Breast cancer (BC) is the most common malignancy diagnosed in women (1 in 8 women) (1). It is also a significant contributor to the overall burden of cancer, accounting for 25% of all new cancer cases (2). Breast cancer is the leading cause of women’s cancer-related death by the metastatic spread of the primary tumor (3). Approximately 3% to 10% of breast cancer patients are diagnosed with metastatic disease at the time of their initial diagnosis (4). While breast cancer has the capacity to metastasize to various organs, reports of metastasis to the urinary bladder are relatively rare (5, 6).
In contrast to the previous BC cases presenting initially with urinary symptoms (which were also confirmed by positive clinical, radiological, and histopathological findings), the aim of this report is to present a case of bladder metastatic BC with absent clinical and radiological features of BC-but positive histopathological findings.
2. Case Presentation
A 67-year-old woman presented with lower urinary tract symptoms (LUTSs), intermittent right flank, and suprapubic pain that had been ongoing for 1.5 years prior to presentation. She was a known hypertensive but controlled with medications. No positive surgical history. There was one episode of microscopic hematuria, but other laboratory investigations (including creatinine, complete blood count, and liver function tests) were normal. Urine cytology was negative for malignancy.
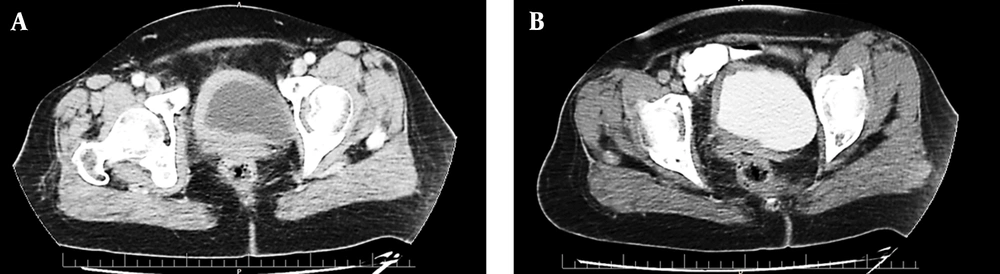
Abdominopelvic ultrasonography revealed an asymmetric diffused bladder wall thickening with a maximum diameter of 14 mm, involving the right lateral and right half of the anterior and posterior wall of the bladder with extension to the right ureteropelvic junction (UVJ), causing mild to moderate hydroureteronephrosis in the right side (pelvic anterior-posterior diameter [APD]: 9 mm). This was later confirmed by a computed tomography (CT) urogram, which also revealed mild right hydronephrosis and mild hydronephrosis of the right kidney and asymmetric bladder wall thickening in the right side of the urinary bladder (Figure 1), in addition to multiple osteoblastic lesions in the bones, suggestive of malignancy. Therefore, a whole-body bone scan was performed, revealing multiple bone lesions in the left sacroiliac (SI) joint, thoracic, and upper lumbar spine.
Computed tomography (CT) scan with intravenous (IV) contrast of the pelvis in the arterial phase (A) and delayed excretory phase (B). An axial section showing asymmetric hyperdense segmental urinary bladder wall thickening involving the right side. The contrast enhancement is 77 Hounsfield units (HU) (from 48 HU before IV contrast injection). There are no signs of involvement of adjacent structures (uterus and adnexa).
In the next step, she had transurethral resection of the bladder tumor (TURBT), revealing a neoplastic mass mainly around the right ureteral site that was deviated from the trigon to the left. A compressive effect had deformed the right ureter to the point that it was impossible to cross a 6 Fr. ureteroscope. The rest of the bladder mucosa was severely pale. Diffuse lesions without a distinct base were resected up to the muscular layer as much as possible.
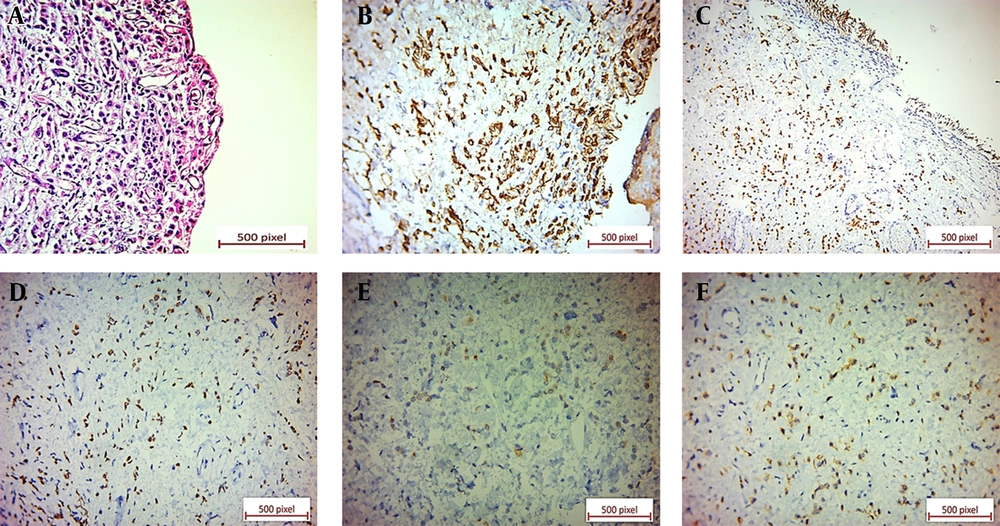
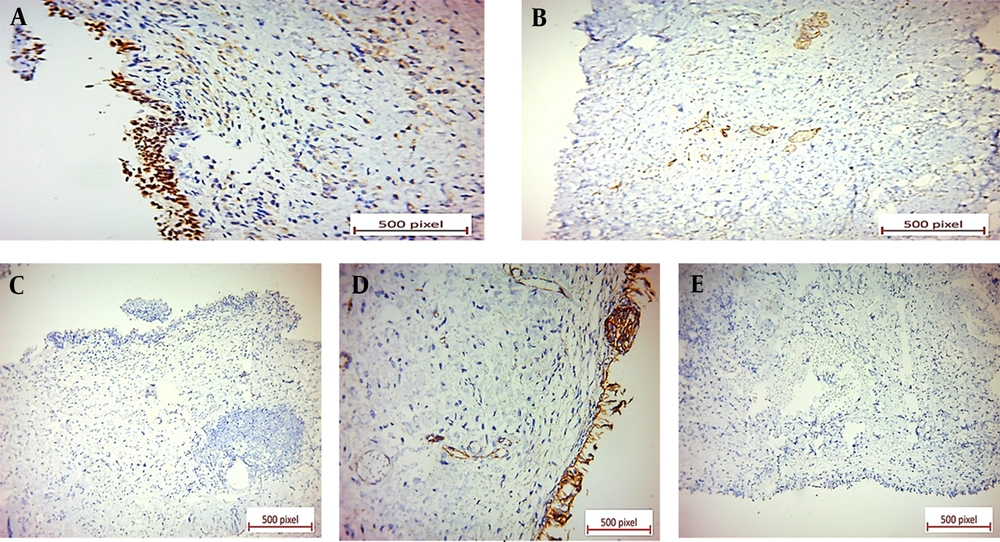
Microscopic examination of the specimen revealed a neoplastic tissue composed of sheets of discohesive single tumoral cells and cords of cells with enlarged, occasionally laterally placed nuclei and pale eosinophilic cytoplasms. A few tumoral cells with signet ring features were also present. The urothelium was ulcerated, and the neoplastic cells diffusely infiltrated the lamina propria and muscularis propria (Figure 2). Immunohistochemical (IHC) staining showed positive immunoreactions for CK7, GATA3, ER, and CD138. Also, GCDFP-15 and CEA were positive in the scattered tumoral cells (Figure 2). P63, E-cadherin, CDX2, B-catenin, and CK20 revealed negative immunoreactions (Figure 3).
(A) Ulcerated urothelium underlined by discohesive tumoral cells with occasional signet ring features (×20). (B) Positive CK7 cytoplasmic immunostaining of tumoral cells in bladder tissue (×40). (C) Positive GATA3 nuclear immunostaining of tumoral cells in bladder tissue (×20). (D) Positive ER nuclear immunostaining of tumoral cells in bladder tissue (×40). (E) Positive GCDFP-15 cytoplasmic immunostaining in a few scattered tumoral cells in bladder tissue (×40). (F) Positive CEA cytoplasmic immunostaining in scattered tumoral cells in bladder tissue (×40).
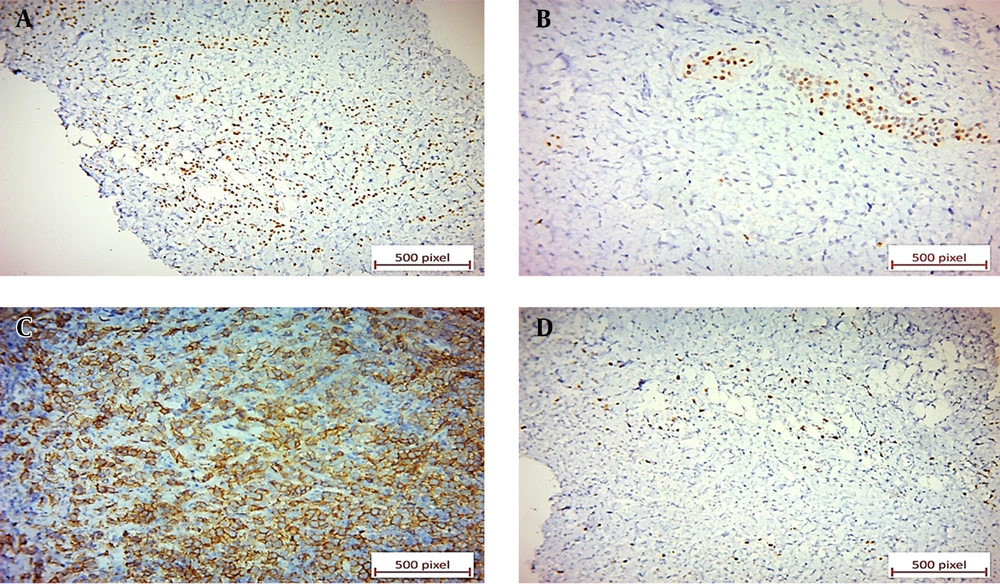
Negative immunoreactions: (A) Negative P63 immunostaining of tumoral cells in bladder tissue. The benign surface urothelium shows positive nuclear staining for P63 (×40). (B) Negative E-cadherin immunostaining of tumoral cells in breast tissue. Some benign breast acini show positive staining (×20). (C) Negative CDX2 immunostaining of tumoral cells in bladder tissue (×20). (D) Negative B-catenin immunostaining of tumoral cells in bladder tissue (×40). (E) Negative CK20 immunostaining of tumoral cells in bladder tissue (×20).
The pathologist reported a carcinoma with plasmacytoid/signet ring features and added a note saying that this tumor would be considered a primary tumor of the bladder if metastasis of lobular carcinoma of the breast could be clinically ruled out.
Physical examination of the breasts and lymph nodes was then performed with no pathological findings. The vaginal and cervical exams and the Pap smear test were also normal. Tumor markers, such as CA 19-9, CA 125, CEA, and CA 15-3, were detected in the normal range. Transvaginal ultrasonography was performed without any additional findings. Both axillary and breast ultrasonography and bilateral mammography were normal, and no enlarged axillary lymph nodes were present (Breast Imaging Reporting and Data System 1 (BI-RADS 1)).
A fluorodeoxyglucose positron-emission tomography/computed tomography (FDG PET/CT) scan from vertex to mid-thigh was requested. In the chest, an asymmetrical soft tissue density nodule in the superolateral quadrant of the right breast with mild metabolic activity was detected. Ipsilateral right axillary adenopathy was also observed, along with right hilar FDG-avid adenopathy. Bilateral subpleural hypermetabolic ground glass nodules were noted in the lung field, suggestive of ground glass metastasis. Moreover, extensive mixed lytic sclerotic bone lesions in the axial and appendicular skeleton were reported.
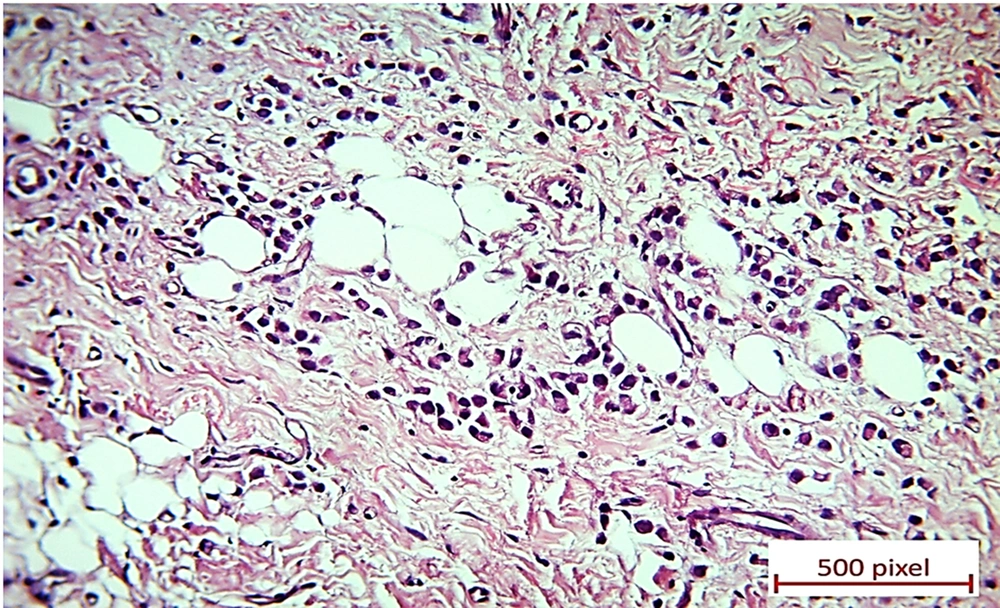
Eventually, an ultrasound sonography-guided core needle biopsy of the right breast nodule and right axillary lymph node was performed. Microscopic evaluation displayed invasive lobular carcinoma of the breast (Figure 4) in the presence of lobular intraepithelial neoplasm (LIN) and lymphovascular invasion (LVI). The right axillary lymph node was also involved, along with the extranodal extension. The tumoral cells were positive for ER, PR, and Her2/neu in the IHC study, and Ki-67 was positive in about 5% - 10% of these cells (Figure 5).
(A) Positive ER nuclear immunostaining of tumoral cells in breast tissue (×20). (B) Positive PR nuclear immunostaining in 1% of tumoral cells in breast tissue. Some benign breast acini are positive for PR (×20). (C) Complete strong membranous HER2 immunostaining in more than 10% of tumoral cells in breast tissue (score 3; ×20). (D) Ki-67 shows nuclear immunostaining of 7% of tumoral cells in breast tissue (×20).
Considering the patient’s preference to preserve her breasts, her age, and the presence of distant metastases, it was decided to start palliative chemotherapy. Today and after 8 courses of palliative chemotherapy with adriamycin, endoxan, and paclitaxel regimens, 12 courses of bone metastasis treatment with Zometa, and daily hormone therapy with letrozole (2.5 mg) in 24-month follow-up, the patient is well, and the urinary symptoms have fully disappeared.
3. Discussion
Secondary tumors are 2% of all bladder neoplasms; the majority of them are due to direct extension (4). Also, BC generally spreads to the lymph nodes, lungs, bones, liver, and skin (7-11).
Clinical presentations of bladder metastasis (BMs) from primary BC in the literature included painless hematuria, LUTSs, and back pain (3, 8, 12). The patients were diagnosed with BC from 7 months to 30 years before the BMs were discovered (7). As our case illustrated, irritative bladder symptoms may also be the first sign of BC. Therefore, investigations, including cystoscopy with taking biopsy samples and imaging studies (such as abdominopelvic ultrasonography, CT scan, and magnetic resonance imaging (MRI)), are highly suggested in such patients.
In most reported cases, BMs from BC were associated with other metastases (5, 12). These findings suggest that BMs are delayed complications of the main disease. Infiltrating lobular carcinoma (ILC, 8% - 14% of BCs) has a more frequent BM in contrast with infiltrating ductal carcinoma (IDC, 65% - 90% of BCs) (9, 11, 13). It is believed that BC spreads to the bladder by retroperitoneal lymphatic involvement, which is more common in ILC patients (6, 7). Our patient also had the lobular subtype of BC.
Infiltrating lobular carcinoma does not often lead to the formation of a definite lump in the breast, and rapid diagnosis by physical examination or mammography may be challenging (13). The most interesting feature of the reported case was the absence of BC in medical history, physical examination, and imaging evaluation (BI-RADS 1), which challenged the pathological findings of bladder biopsy with respect to other data.
Furthermore, the patient evaluation must include an assessment of the serum CA15-3 level, which is the most sensitive tumor marker in BC follow-up. Its essential function is to observe the patient’s treatment response and cancer recurrence (14). However, in our reported case, all tumor markers were normal.
Although the cost-effectiveness of the PET/CT scan in BC patients is still being debated: it may be helpful in locating other metastatic sites (15). The 18F-FDG PET/CT scan helped us to detect suspicious sites for taking biopsies.
According to the literature, bladder metastatic BC patients have a very limited survival after the onset of urinary symptoms (between 1 month to 2 years), though few articles have reported survival up to 5 years (8, 10). Nevertheless, the general condition of our patient is favorable, and she is disease-free after 24 months of follow-up.
3.1. Conclusions
Even though the incidence of BMs from BC is rare and the diagnosis relies on pathologic evaluation and immunohistochemical staining, the reported case confirms that urinary symptoms due to BMs from BC may be the first expression of the primary disease. Thus, metastatic BC may rarely initially present with irritative lower urinary symptoms and absent clinical and radiological features of BC-but positive histopathological findings. The assessment of patients with a urinary symptom history should consider the possibility of BMs. Evaluations performed on these patients should include blood sampling, urine analysis and cytology, radiologic imaging, cystoscopy, and biopsy if bladder involvement is doubtful.