1. Background
With nearly 430,000 new cases each year, bladder neoplasm is the ninth most prevalent malignancy. Men have three times higher bladder neoplasm incidence than women. About 30% of patients have muscle invasion, which indicates rapid progression, metastasis, and a poor outcome, while 70% of patients have no muscle invasion. The prognosis for bladder neoplasm patients has not changed significantly over the past few decades despite surgery and adjuvant therapies, with 50% of patients dying from cancer (1). In addition to conventional urothelial carcinoma (UC), there are more than a dozen microscopically unique forms of bladder cancer described in the recent World Health Organization (WHO) classification. Squamous and glandular differentiations are 2 common findings in otherwise conventional UC and have uncertain clinical impacts. On the other hand, the development of sarcomatoid, small cell, micropapillary, and plasmacytoid variants (2). The histologic grade and pathologic stage of the tumor are factors in bladder neoplasm prognosis. Muscle invasion is the most important factor in bladder neoplasms for disease-free survival and outcome. Radical cystectomy is the main therapy for bladder tumors involving muscle invasion, but the 5-year survival rate is still dismal. Additionally, neoplasms that are not muscle-invasive can relapse and turn into muscle-invasive cancer (3). Adjuvant therapies are only effective for a small percentage of bladder neoplasm patients, which is one of the main reasons for the high mortality rate among these patients. To improve prognosis and disease-free survival, new target therapies must be developed (4). Numerous organizations have provided a thorough description of the disease's genetic and epigenetic changes over the past ten years. Our understanding of bladder cancer biology has changed as a result of these developments, which have also led to the development of novel therapeutic hypotheses, given us the first chance to comprehend chemoresistance, and identified new chemotherapy targets. To find new target therapies for a better prognosis, it is necessary to explain the molecular mechanisms of bladder neoplasms. The transmembrane glycoprotein EGFR belongs to the family of ErbB receptors (5). The EGFR gene, which is associated with cell proliferation, is located on chromosome band 7 p12 (6). Tumors from the bladder, breast, colon, and lung have all been found to express the EGFR gene (7). Tumor growth, angiogenesis, and prognosis are all affected by high EGFR expression. Therefore, EGFR expression may affect tumor characteristics, as seen in histopathology. Target therapy also depends on the evaluation of EGFR expression in tumor tissue (8). Since trastuzumab, an anti-ERBB2 antibody, was approved for the treatment of breast cancer in 1998, several selective ERBB inhibitors have been developed to treat a variety of cancers with EGFR overexpression or mutation. According to various histopathological subtypes, some studies on bladder neoplasms found that EGFR overexpression varied from 27 to 74 percent (9). Therefore, it is necessary to assess how frequently EGFR expression occurs in each community.
2. Objectives
The purpose of our study was to determine the level of EGFR expression in bladder neoplasms and the relationship between tumor characteristics and EGFR expression.
3. Methods
3.1. Study Population
Our investigation focused on 30 bladder neoplasm tissues and was cross-sectional. Malignancies detected during cystoscopy and verified by a pathology report were inclusion criteria. Patients who had received neoadjuvant therapy before surgery were excluded from consideration. We conducted our study on all patients who met these requirements and were sent to Tehran's Sina Hospital between 2020 and 2022. Additionally, patients without access to clinical data or those whose samples were insufficient or unsuitable for the tests were excluded from the study. One issue with time-limited studies is the number of patients needed to participate. Therefore, extending the time frame resolves the issue.
3.2. Study Assessments
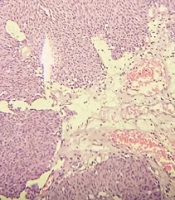
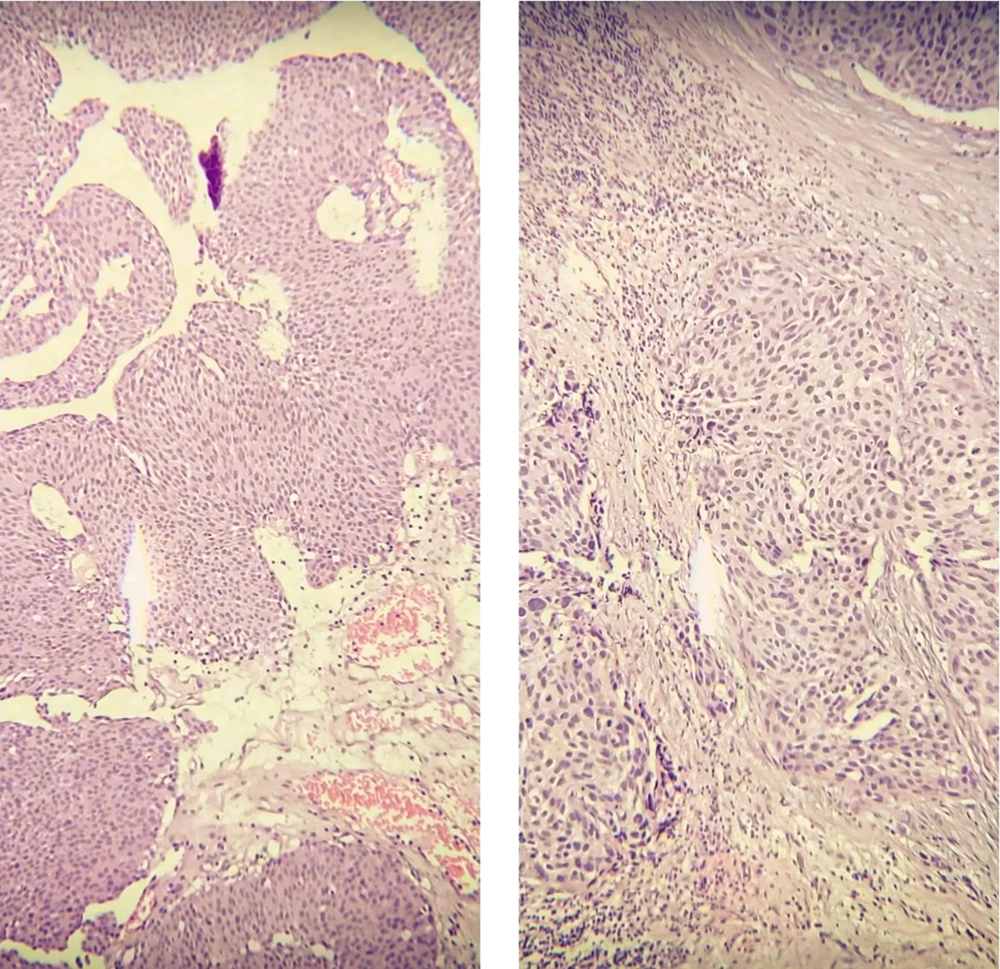
Following surgery, tissue was received and examined histopathologically to determine the presence of a bladder neoplasm. Neoplastic areas were removed and fixed in 10% formalin. These cancerous specimens were then turned into paraffin blocks. Hematoxylin and Eosin (H and E) stained slides for pathological evaluation of tumor size, presence or absence of lymphovascular invasion, tumor focality, histologic tumor grade, and pathologic stage (Figure 1). G1, G2, and G3 were the different tumor grades (10), and according to WHO classification, (11) WHO grade 1 lesions that show slight cytologic atypia and mitoses are diagnosed in the WHO/ISUP system as low-grade papillary urothelial carcinomas. WHO grade 2 is a very broad category. It includes relatively bland lesions, which in some places are diagnosed as WHO grade 1 to 2; these lesions in the WHO/ISUP system would be called low-grade papillary urothelial carcinoma. In other cases, WHO grade 2 lesions border on higher-grade lesions, which in many institutions are called WHO grade 2 to 3; these lesions in the WHO/ISUP classification system would be called high-grade papillary urothelial carcinoma.
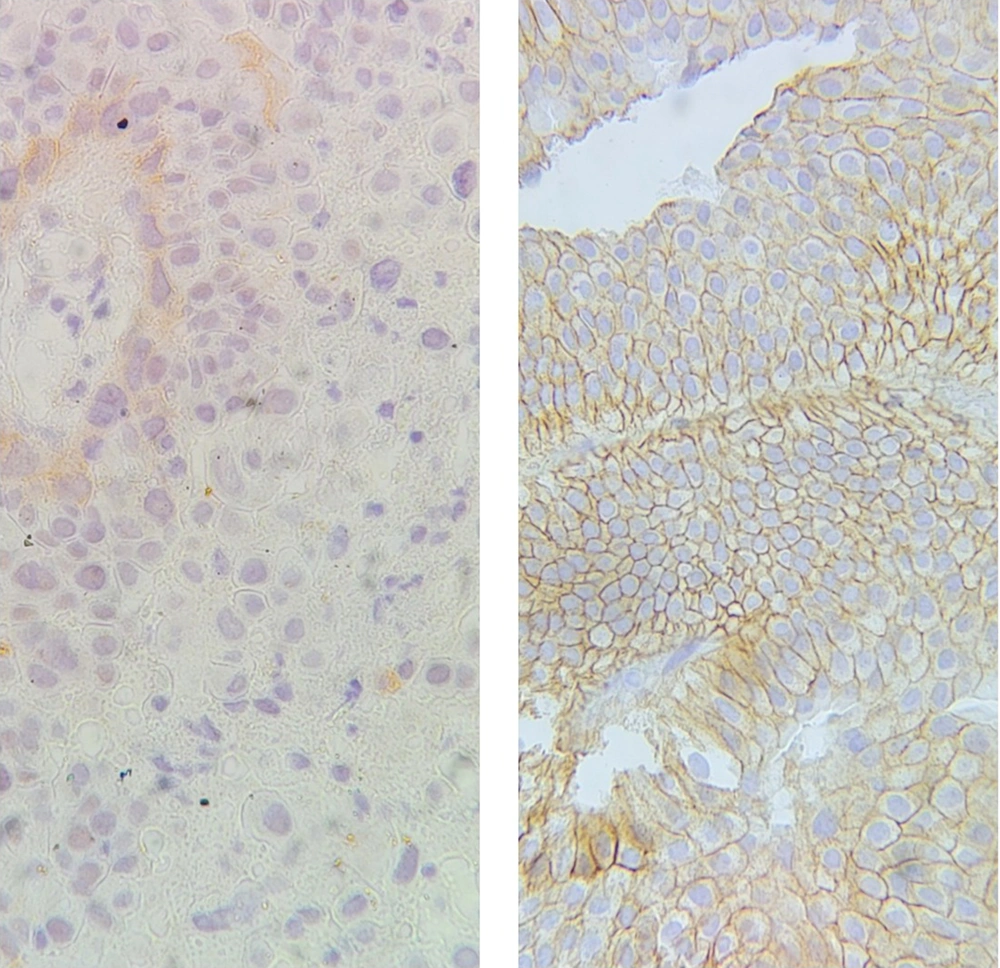
Regarding bladder cancer prognosis, the pathological stage is one of the most crucial variables. For patient management, accurate staging is essential. According to the TNM (tumor, lymph nodes, and metastasis) staging system, pT1 tumors are those that have invaded the lamina propria but not the muscularis propria; pT2 tumors as those invading the muscularis propria; pT3 tumors as those invading perivesical tissue; and pT4 tumors as those invading other organ structures (prostate, uterus, vagina, pelvic wall, or abdominal wall) (12). To evaluate the gene expression level on prepared paraffin blocks, immunohistochemistry (IHC) staining for EGFR was also carried out. EGFR expression in bladder neoplasm samples was evaluated and compared with normal bladder samples from random biopsies for other purposes (Figure 2).
3.3. Immunohistochemical Analysis
Sina Hospital's pathology department archives each patient's tumors. To precisely mark areas of the tumor, two experienced pathologists examined the H and E-stained sections of these masses. To determine EGFR expression, IHC was conducted on 3-μm sections that had been paraffin-fixed and paraffin-embedded. A rabbit anti-EGFR monoclonal antibody (1:50 dilution, duplicate EP22; Master Diag'ostica, Spain) was used to examine EGFR expression. Sections were cut from each block, deparaffinated with xylene, rehydrated by a sequence of decreasing ethanol concentration, and then recovered for antigen in CC1 cytostatic solution (pH 7.4.95°C; System Y. Ventana) for 60 min and the final time of all recovered solutions was cooled for at least 30 min at room temperature (25°C). Previously, to inhibit endogenous peroxidase, slides were treated for 10 min with 3% H2O2 in methanol. Slides were incubated with monoclonal antibodies for 120 min at room temperature and stained with DAKO's Envision detection system. The invention of the color reaction was established with 3,3′-diaminobenzidine tetrahydrochloride (DAB plus; DAKO Glostrup, Denmark) as the substrate, and nuclear contrast was achieved with hematoxylin/water contrasted with ammonia. Formal, paraffin-embedded fixed sections of normal prostate tissue were used as positive controls for EGFR. Negative controls were performed by replacing the primary antibody with PBS/non-immune mouse serum. EGFR expression staining was scored on a scale of 0 - 4 (from no staining to strong staining) (13). Scores +2 to +4 were considered positive expressions.
3.4. Statistical Analysis
The statistical analysis was conducted using IBM's SPSS version 23.0 for Windows, and the results were summarized as rates (percentages) for variables. The chi-square test was used to link unqualified variables, while the t-test or Mann-Whitney U test was used to link quantitative variables between subgroups. P-values ≤ 0.05 are regarded as statistically significant.
4. Results
In our study, 30 patients consisting of 24 males and 6 females with bladder neoplasms were included for EGFR expression assessment. Baseline parameters are shown in Table 1. Regarding age subgroups, 13.4% were aged lower than 55 years, 36.6% were aged 55 to 65 years, 30.0% were aged 66 to 75 years, and 20.0% were aged higher than 75 years. Regarding tumor staging, the muscular layer and closed soft tissue invasion was revealed in 20.0% and 3.4%, respectively. In all subjects, the tumors had a single pattern (papillary urothelial neoplasm). Regarding tumor grading, 53.3% were low grade, and 46.7% were high grade. In addition, 13.4% is sized higher than 5cm (Table 2).
| Variables | No. (%) |
|---|---|
| Gender | |
| Female | 6 (20) |
| Male | 24 (80) |
| Age, y | |
| < 55 | 4 (13.4) |
| 55 - 65 | 11 (36.6) |
| 65 - 75 | 9 (30) |
| > 75 | 6 (20) |
Demographic Data
| Tumor’s Characteristics | No. (%) |
|---|---|
| Type of tumor surgery | |
| Total cystectomy | 2 (6.7) |
| Partial cystectomy | 0 (0.0) |
| TUR-BT | 28 (93.3) |
| Presence of lymphovascular invasion | 3 (10.0) |
| Tumor size, cm | |
| < 2 | 12 (40.0) |
| 2 to 5 | 14 (46.6) |
| > 5 | 4 (13.4) |
| Tumor grade | |
| Low | 16 (53.3) |
| High | 14 (46.7) |
| Tumor staging | |
| Without invasion (Ta) | 10 (33.3) |
| Invasion to lamina propria (T1) | 13 (43.3) |
| Invasion to muscularis propria (T2) | 6 (20.0) |
| Invasion to perivesical soft tissue (T3) | 1 (3.4) |
| Tumor multiplicity | |
| Single | 30 (100) |
| Multifocal | 0 (0.0) |
Baseline Characteristics of Bladder Neoplasms
Overall, EGFR was expressed in 25 (83.3%) patients. EGFR expression frequencies were a score of 0, 1 (3.33%), a score of 1, 4 (13.33%), a score of 2, 3 (10%), a score of 3, 10 (33.33%), and a score of 4, 12 (40%). Scores of 2 and more than 2 are considered positive. In male patients, 18 (75%) and 6 (100%) showed positive staining EGFR in female patients. Low-grade tumors showed in 14 (87.5%) patients, and high-grade 10 (71.4%) patients had positive EGFR staining. According to Table 3, there was no connection between EGFR expression and demographic data and histopathological features, including sex (P-value = 0.359), age (P-value = 0.659), surgery type (P-value = 0.39), lymphovascular invasion (P-value = 0.685), tumor size (P-value = 0.294), histologic tumor grade (P-value = 0.171), and pathologic stage (P-value = 0.957).
| Characteristics | EGFR (+) | EGFR (-) | P-Value |
|---|---|---|---|
| Gender | 0.359 | ||
| Male | 18 (75) | 6 (25) | |
| Female | 6 (100) | 0 (0.0) | |
| Mean age, y | 67.7 | 61.1 | 0.659 |
| Vascular invasion | 0.685 | ||
| With invasion | 2 (66.6) | 1 (33.3) | |
| Without invasion | 22 (81.4) | 5 (18.5) | |
| The mean size of the tumor, cm (mean ± SD) | 2.7 ± 1.8 | 3.5 | 0.39 |
| Tumor grade | 0.171 | ||
| Low | 14 (87.5) | 2 (12.5) | |
| High | 10 (71.4) | 4 (28.5) | |
| Tumor staging | 0.957 | ||
| Without invasion (Ta) | 8 (80) | 2 (20) | |
| Invasion to lamina propria (T1) | 11 (78.5) | 3 (21.4) | |
| Invasion to muscularis propria (T2) | 5 (100) | 0 (0.0) | |
| Invasion to perivesical soft tissue (T3) | 0 (0.0) | 1 (100) |
Comparing Baseline Characteristics in the Groups with and Without EGFR Expression a
5. Discussion
The shortcomings of imaging techniques and histopathological analysis drive the need for new biomarkers in assessing aggressive behaviors of cancers. Some biomarkers have been developed to evaluate the behavior of bladder neoplasms, and strong data has been obtained from these biomarkers to estimate survival. However, there is still disagreement over whether these biomarkers can accurately predict the aggressive behavior of tumors or serve as the best method for predicting prognosis and survival. When there is no muscle invasion in a bladder neoplasm, treatment is simpler (14). EGFR expression in the normal bladder is in the basal layer of urothelium and relates to less differentiated cells. So, the role of EGFR expression in urothelial cell differentiation is not unexpected, and high EGFR expression in bladder neoplasms has been reported (15). In our study, we evaluated EGFR expression in bladder neoplasms of the local population and found that EGFR expression was present in 83.3% of bladder neoplasm patients. The results of our study also showed no evidence of a significant correlation between EGFR expression and prognostic factors like muscle invasion, histologic tumor grade, or pathologic stage. However, it is suggested that the EGFR marker is one of the important biomarkers in the growth of bladder neoplasms (16). Additionally, EGFR expression, which is associated with a poor prognosis, is present in 70% of bladder neoplasms with muscle invasion (17). However, it appears that the expression of EGFR suggests a poor prognosis, and thus, impaired activity of these growth factor receptors may contribute to the prognosis of cancer patients (18). Patients with EGFR expression who have never received neoadjuvant therapy can use EGFR inhibitors (15). Recent studies have described how EGFR expression is present in bladder neoplasms and how the expression score relates to the grade, stage, and outcome of the histology (19). However, we discovered no connection between EGFR expression and the aforementioned results. A study by Mason et al. revealed EGFR pathway genetic variation affects the bladder neoplasm's prognosis and mortality. Understanding these molecular factors affecting bladder neoplasm survival may help with target therapy, cancer prevention, and new treatments (20). Research conducted by Chaux et al. found EGFR expression in 74% of bladder cancers, but we showed EGFR expression in 83.3% of patients. Because EGFR mutations in some exons may be missed in paraffin section examination, IHC staining for EGFR may not help predict response to EGFR inhibitors and need supplementary studies (21). The proliferation, migration, recurrence, and metastasis of neoplastic cells are linked to EGFR expression. Therefore, tumor grade and stage are related to EGFR expression in bladder neoplasms (22). A study by Wang et al. showed larger tumor size, higher histologic tumor grade, and lymphovascular invasion significantly correlated with high EGFR expression. These findings suggested a connection between EGFR expression and aggressive histopathological characteristics (23). But our data showed no significant relationship with these features in our population (P-value > 0.05). Research conducted by Kim et al. showed the degree of EGFR expression is a new prognostic biomarker to estimate the effectiveness of target therapy in patients with recurrence or metastasis. EGFR was a significant biomarker of bladder neoplasms with progression (24). Research conducted by Nicholson et al. study described breast, stomach, uterus, and colorectal cancers may not all have EGFR as a significant prognostic biomarker. However, it might be significant for prognosis in other cancers (25). A study by Badawy et al. showed EGFR expression in 86% of bladder neoplasm with the same method, which was in line with our findings. They discussed the significant correlation between EGFR expression, tumor histologic grade, and pathologic stage and demonstrated a connection between EGFR expression and schistosomal-associated bladder cancer (26). Due to the small population size and the low incidence of schistosomal bladder cancer in our society, our data did not show any significant correlation. Recent analyses of bladder neoplasms at the molecular level revealed that basal-squamous-like subtypes had poor prognoses and higher EGFR expression (27). Therefore, EGFR expression varies depending on the kind of neoplasm and could be a prognostic indicator of this type. Additionally, urinary EGFR measurement may be a quick and useful test for evaluating the prognosis of bladder neoplasms (28). Uncertainty still exists regarding the way that EGFR expression works and how it relates to unfavorable results. The relationship between aggressive behaviors, EGFR expression, and the prognosis of bladder neoplasm varies greatly across social groups. Therefore, this biomarker's value in predicting tumor behavior is societally relevant. The patient's race or the specific antibodies used for IHC staining may be related to this (29). These new medications are being tested in numerous trials on patients with bladder cancer alone or with chemotherapy. The role of novel targets and prognostic hints that can direct the most effective treatment choice for advanced disease is defined by a better understanding of the molecular biology of urological malignancies. The small sample size and only one significant research center were our limitations. Therefore, we advise conducting additional research using large sample sizes and multicentric populations.
5.1. Conclusions
Bladder cancer cells that exhibit aggressive behaviors quickly grow and spread, necessitating immediate and aggressive treatment. Stage, grade, and specific genetic mutations in the tumor are a few variables that can influence aggressive behavior. The role of EGFR expression in tumorigenesis and cancer development is a hot topic for research. There needs to be more research done in each society regarding EGFR as a prognostic biomarker.