1. Introduction
The coronavirus disease 2019 (COVID-19) and cytomegalovirus (CMV) disease are opportunistic co-infections of each other in immunocompetent and transplant recipients (1-5). Numerous studies have observed CMV reactivation following severe COVID-19. Additionally, CMV has been the most common co-infection among fatal cases of severe COVID-19 lung infection in Indian renal transplant recipients (6). However, there is limited literature on the association of CMV reactivation with mild COVID-19 infection. We present 2 cases of CMV reactivation after mild COVID-19 infection in renal transplant recipients during the fourth wave of the pandemic in India. Subsequently, we briefly review the literature and propose a few research questions for future longitudinal studies.
2. Case Presentation
2.1. Case 1
A 30-year-old man with end-stage primary lupus nephritis underwent mother-to-son kidney transplantation 1 year after the initiation of hemodialysis. Serology tests conducted immediately before the transplant indicated the presence of immunoglobulin G (IgG) antibodies to CMV in the recipient. The donor also tested positive for CMV IgG antibody. With a 3/6 human leukocyte antigen (HLA) mismatch, the patient received anti-T lymphocyte globulin as induction therapy. He was started on tacrolimus, mycophenolate mofetil, and prednisone for maintenance therapy. The cold ischemia time was 28 minutes. The patient had an uneventful postoperative stay and was discharged on the sixth postoperative day with a baseline creatinine of 1.8 mg/dL. He was regularly followed up. After 3 months, he was admitted with complaints of fever for 4 days and had a sore throat and cough but no derangement of renal function. He tested positive for COVID-19 infection and was managed conservatively on an outpatient basis. Seven days later, he was readmitted with chief complaints of new-onset loose stools, abdominal pain, and back pain. On physical examination, the patient had tachycardia of 110 beats per minute, blood pressure of 120/78 mm Hg, and a respiratory rate of 16/minute with normal oxygen saturation at room air. On auscultation, there were normal bilateral breath sounds, and normal heart sounds. Abdominal examination was normal. Laboratory investigations revealed mildly raised creatinine and leukopenia with a white blood cell (WBC) count of 2400/µL. The CMV polymerase chain reaction (PCR) test detected 128 500 copies/mL. The patient was admitted and treated with an injection of ganciclovir (500 mg/day) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Mycophenolate mofetil was discontinued because of CMV infection. There was a subsequent increase in leukocyte count (WBC 4500/μL) on day 5 of admission, with relief of gastrointestinal symptoms. However, the patient complained of persistent back pain and had a gradual decline in graft and renal function. With decreasing urine output, dialysis was initiated. Subsequently, the patient developed sudden-onset altered sensorium and had a fatal cardiac arrest.
2.2. Case 2
A 34-year-old man with end-stage chronic nephritis underwent cadaveric kidney transplantation with a 3/6 human leukocyte antigens (HLA) mismatch. Pretransplant serology indicated the presence of IgG CMV antibody in the recipient with an unknown seronegativity status in the donor. For induction of immunosuppression, anti-thymocyte globulin was used with maintenance therapy, including tacrolimus, mycophenolate mofetil, and steroid. The cold ischemia time was 3 hours. The patient had delayed-onset graft function with a baseline creatinine of 2 mg/dL on the tenth postoperative day.
The patient had uneventful follow-ups until the fifth month, when he was admitted due to declining renal function. Creatinine was 3.6 mg/dL. He had normal blood counts, sterile urine culture, and proteinuria, with a urine protein creatinine ratio (UPCR) of 0.9. The CMV PCR was negative. Subsequent kidney biopsy revealed active antibody-mediated rejection, and the patient was managed with 6 cycles of plasmapheresis, which increased the kidney function (creatinine of 3 mg/dL).
One month later, he was admitted with a fever and cough. The patient tested positive for COVID-19 infection and was managed conservatively on an outpatient basis. Within 10 days, he developed multiple episodes of hematochezia, pain in the abdomen, and diarrhea. Physical examination revealed that the patient had tachycardia of 120 beats per minute, blood pressure of 104/64 mm Hg, and a respiratory rate of 20/minute with normal oxygen saturation at room air. Bilateral breath sounds were normal. The abdomen was soft, non-tender, and had increased peristaltic sounds. Urine output was maintained with stable creatinine. A stool routine and microscopic examination revealed numerous red blood cells (RBCs) and few pus cells; however, no parasite was detected. A repeat CMV PCR was performed, which returned a positive result with a viral load of 3000 copies/mL. The patient had normal blood cell counts. The patient was admitted and treated with an injection of ganciclovir (500 mg/day) for 7 days, and mycophenolate mofetil was discontinued. With relief of gastrointestinal symptoms, he was discharged on the eighth day when he was switched to oral valganciclovir at 450 mg/day instead of ganciclovir injections. He remained on weekly follow-up. There was initial mild deterioration in kidney function, with creatinine increasing from 3 to 3.5 mg/dL. He remained afebrile, and the general condition gradually improved with no further episodes of hematochezia. The patient's renal function gradually improved over time, with a gradual decline in creatinine levels from 3.5 mg/dL to the baseline level of 3.0 mg/dL. A repeat CMV PCR conducted after 30 days returned a negative result.
3. Discussion
The association between COVID-19 infection and CMV reactivation has been widely studied during the pandemic. In a study conducted in France on 38 patients on mechanical ventilation in the intensive care unit (ICU) after acute respiratory distress syndrome (ARDS) related to COVID-19 infection, 47% showed evidence of CMV or herpes simplex virus (HSV) reactivation (7). A similar Japanese study on 26 patients admitted to the ICU with severe COVID-19 pneumonia showed that 23% of the patients developed CMV viremia (8). A study conducted in a tertiary care hospital in India showed that CMV reactivation occurred in 38% of the patients admitted to the ICU after COVID-19 ARDS and was associated with 100% mortality (9). Prolonged mechanical ventilation, corticosteroid use, and lymphopenia have been identified as risk factors that lead to CMV reactivation after COVID-19 infection in immunocompetent patients. Other pathological events, including M1 polarization of macrophages and inflammatory cytokines (such as tumor necrosis factor α), have been postulated as possible causes for CMV reactivation after COVID-19 infection (8).
Due to immunosuppression, there is reactivation of CMV infection in more than 30% of postrenal transplant patients in India (6). This raises the possibility of a very high risk of CMV infection activation in these patients if there is severe COVID-19 infection. In an Iranian case series study, out of 10 postrenal transplant patients who had severe COVID-19 infection, 4 patients subsequently developed CMV infection with 50% fatality (10). In a case-control study from India, among fatal cases of COVID-19 infection in postrenal transplant recipients, CMV was the most common co-infection (6). These recently published studies suggest that in postrenal transplant recipients with severe COVID-19 infection, CMV reactivation is common and is associated with high mortality. However, the severity of COVID-19 manifestations decreased during the fourth wave of COVID-19 infection, and there is a lack of published data on the incidence of CMV reactivation after mild COVID-19 infection in renal transplant recipients.
However, interestingly, several case studies have reported cases of CMV reactivation in temporal association with COVID-19 vaccination during the last year, with patients presenting with diverse clinical manifestations from CMV myocarditis to CMV proctitis (11-13).
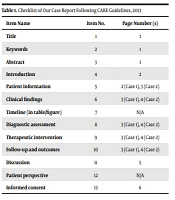
We reported 2 cases of CMV infection following mild COVID-19 infection in renal transplant recipients following the CARE (Case Reports) guidelines (Table 1) after securing appropriate informed consent (14).
| Item Name | Item No. | Page Number (s) |
|---|---|---|
| Title | 1 | 1 |
| Keywords | 2 | 1 |
| Abstract | 3 | 1 |
| Introduction | 4 | 2 |
| Patient information | 5 | 2 (Case 1), 3 (Case 2) |
| Clinical findings | 6 | 3 (Case 1), 4 (Case 2) |
| Timeline (in table/figure) | 7 | N/A |
| Diagnostic assessment | 8 | 3 (Case 1), 4 (Case 2) |
| Therapeutic intervention | 9 | 3 (Case 1), 4 (Case 2) |
| Follow-up and outcomes | 10 | 3 (Case 1), 4 (Case 2) |
| Discussion | 11 | 5 |
| Patient perspective | 12 | N/A |
| Informed consent | 13 | 6 |
Abbreviations: N/A, not available.
In both cases, CMV infection was treated by discontinuing mycophenolate mofetil and administering ganciclovir injections, which resulted in 2 contrasting clinical outcomes.
Several important clinical questions are raised by our 2 cases of CMV infection following mild COVID-19 infection in renal transplant recipients during the fourth wave in India, along with the growing body of literature over the past two years on the increased risk of CMV infection following COVID-19 infection and vaccination:
1. Does mild COVID-19 infection without ICU admission raise the risk of CMV reactivation in renal transplant recipients?
2. Would screening for CMV reactivation be an effective strategy for detecting early CMV reactivation in postrenal transplant patients after mild COVID-19 infection? If such screening is used, should routine screening be used for all renal transplant recipients, or should certain selective patients with certain risk factors (e.g., lymphocyte depletion and graft dysfunction) be screened?
3. Does CMV reactivation that follows mild COVID-19 infection have a poorer prognosis than CMV reactivation without preceding COVID-19 infection in renal transplant recipients?
These clinical questions can be answered through longitudinal follow-up studies. With the assumption that the COVID-19 virus is expected to last in the community for the next decade, it is probably the right time to adapt to the new normal by addressing these questions.
Based on the above-mentioned clinical and research questions, we suggest the following research design for a future follow-up study (Table 2).
| Study Design | Longitudinal Prospective Study |
|---|---|
| Aim | To find the incidence and risk factors of CMV reactivation after mild COVID-19 infection in renal transplant recipients and to evaluate the prognosis. |
| Primary objective | To find the incidence of CMV reactivation within 3 months of mild COVID-19 infection in renal transplant recipients. |
| Secondary objective 1 | To find the prevalence of risk factors of CMV reactivation, e.g., lymphocyte depletion, graft dysfunction, and pretransplant recipient CMV serostatus in renal transplant recipients after mild COVID-19 infection, and to see if any of these risk factors are positively correlated with CMV reactivation. |
| Secondary objective 2 | To find clinical outcomes of patients with CMV reactivation following COVID-19 infection with endpoints, e.g., clinical recovery, negative CMV PCR, return of creatinine to the baseline level, and death within 3 months of CMV reactivation. |
| Study methodology | After receiving ethical approval from the appropriate authority, renal transplant recipients with mild COVID-19 infection may be followed for 3 months by CMV PCR tests every 2 weeks, and if CMV is detected, they may be followed for the clinical outcome for 3 months. |
Abbreviations: CMV, cytomegalovirus; PCR, polymerase chain reaction.