1. Background
Thalassemia is a group of inherited genetic blood disorders passed down in an autosomal recessive manner that affects the body’s ability to produce hemoglobin and red blood cells. This disease occurs due to gene mutations giving rise to alpha or beta globin chain production, resulting in ineffective erythropoiesis and severe hypochromic microcytic anemia (1).
Beta-thalassemia is most common in regions like the Mediterranean, Middle East, Central Asia, and parts of China, with Cyprus, Sardinia, and Southeast Asia having the highest carrier rates of up to 50%. Its prevalence is historically linked to protection against malaria. Despite its spread to regions like Northern Europe due to migration, data on carrier rates remain limited. Of the global 1.5% carriers (80 - 90 million people), about 60,000 symptomatic births occur annually, mostly in developing countries. The Thalassemia International Federation reports that only 200,000 patients receive regular treatments. It is estimated that 5 - 7% of the world’s population are carriers of a thalassemia gene (2).
Patients with thalassemia major depend on chronic blood transfusions for survival. Each filled milliliter of red blood cells increases iron intake by 1 mg in Thalassemia Major (TM) patients receiving blood (3). Consequently, one of the enduring effects of frequent blood transfusions is iron overload, which heightens the susceptibility to heart disease, liver disease, and endocrine disorders. The discovery of iron-chelating drugs has improved patients’ life prospects over the past few decades. Iron-chelating Drugs (ICDs) remove excess iron from the body and reduce iron accumulation in tissues (4). There are currently three ICDs available for clinical use: (1) Deferoxamine (with its limited oral absorption, necessitates its administration through subcutaneous, intravenous, or intramuscular routes); (2) deferiprone; and (3) Deferasirox (5).
Deferasirox (DFX) is available in various formulations, including Exjade, Jadenu, and Nanojade. Jadenu and Nanojade tablets represent new DFX formulations in the Iranian pharmaceutical market. Exjade must be dissolved in fluids before taking, but Nanojade and Jadenu can be taken in a single step, with or without a light meal (6). Research findings indicate that using ICDs is associated with an elevated risk of renal failure, including kidney stones and hypercalciuria, though the precise reasons for this link remain unknown (7, 8).
Deferasirox is a potent and specific oral chemical compound belonging to the class of N-substituted bis-hydroxyphenyl-triazole (9). The FDA approved this drug as a primary treatment for transfusion-related iron overload in 2005, and the European Medicines Agency (EMA) followed suit in 2006. Since its approval, more than 150,000 patients per year have used this drug (10).
Deferasirox renal toxicity can have several clinical manifestations that indicate a decrease in Glomerular Filtration Rate (GFR) or dysfunction of the proximal tubule, and an increase in the level of serum creatinine was the most common side effect in previous studies (11).
Proximal tubulopathy refers to a group of disorders that affect the proximal tubules, essential components of the kidney responsible for reabsorbing vital substances from the urine. Various factors, including genetic abnormalities, exposure to certain medications, toxins, or systemic diseases, can cause proximal tubulopathy. Inherited disorders such as Dent disease and Fanconi syndrome can disrupt normal proximal tubule function. Additionally, certain medications like Deferasirox have been associated with proximal tubular damage. The clinical presentation of proximal tubulopathy can vary based on the underlying cause and the extent of tubular dysfunction. Common symptoms include excessive thirst (polydipsia), increased urine volume (polyuria), electrolyte imbalances (such as low potassium or phosphorus levels), acid-base disturbances, and signs of renal Fanconi syndrome (such as glycosuria, proteinuria, aminoaciduria, and phosphaturia). Diagnosis involves a thorough medical history review, physical examination, laboratory tests, and imaging studies to evaluate kidney function and identify the underlying cause (12).
Vitamin E (alpha-tocopherol) is a powerful fat-soluble antioxidant that protects cells from oxidative stress, regulates immune system function, maintains endothelial cell integrity, and balances normal coagulation (13).
In various studies on treating Acute Kidney Injury (AKI) caused by nephrotoxicity, the administration of vitamin E has shown significant results. These studies have demonstrated a decrease in serum levels of Cr and BUN, as well as a reduction in the level of MDA in kidney tissues (14-16). However, there is still debate about the effects of vitamin E on kidney function.
2. Objectives
This study aimed to investigate the potential effect of vitamin E in preventing acute kidney injury and proximal tubulopathy in thalassemia major patients undergoing treatment with Deferasirox (Nanojade).
3. Methods
3.1. Patients and Design
The present double-blind, prospective, randomized, controlled clinical trial was registered in the Iranian Registry of Clinical Trials (IRCT) with the registration number IRCT20221111056466N1. The trial was conducted at Amirkabir Hospital in Arak, Iran, from December 2022 to February 2023.
Before participating in the trial, all patients or their surrogates provided written informed consent as part of the enrollment process. Throughout the study, strict adherence to the guidelines set by the Consolidated Standards of Reporting Trials (CONSORT) was followed, ensuring comprehensive and accurate reporting of the trial’s findings.
We enrolled male and female patients with thalassemia major, aged 14 - 60, with no other concurrent medical conditions. The exclusion criteria included patients with a recent history of pregnancy, organ failure, alcohol or substance abuse, and those who did not provide consent.
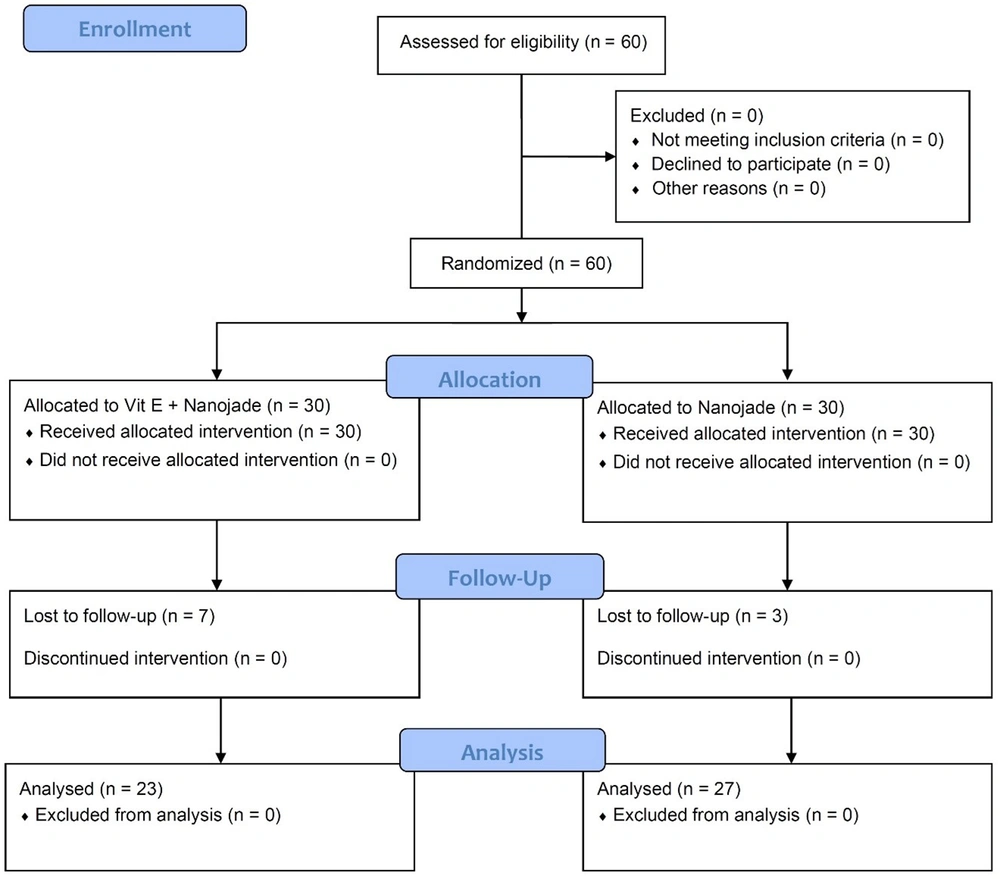
We employed a computer-generated simple randomization method to assign patients to the control or vitamin E groups, maintaining a 1: 1 ratio. Each group consisted of 30 participants, and the researchers, clinicians, patients, and families were blinded to their group assignments, ensuring equitable allocation. During the follow-up period, three patients from the control group and seven from the vitamin E group were lost to follow-up (Figure 1). The trial was designed by the authors who collected and analyzed the data, verified the accuracy and completeness, and vouched for the fidelity of the trial to the protocol.
3.2. Trial Interventions
For the intervention group, in addition to the standard treatment, individuals with ferritin levels above 1,000 receive oral iron chelator Deferasirox (brand name Nanojade, manufactured by SinaGen, Iran) at a dose of 20 mg/kg and vitamin E (brand name E-Zavit 400, manufactured by Zahravi, Iran) once daily for 30 days, while fasting.
The control group received only the standard treatment, including oral iron chelator Deferasirox (brand name Nanojade, manufactured by SinaGen, Iran) at 20 mg/kg for individuals with ferritin levels above 1,000 while fasting.
3.3. Measurements and Endpoints
In this study, various factors were measured at different time points to assess the efficacy and outcomes. Baseline measurements were taken, and subsequent measurements were obtained on days 7 and 30.
At baseline and on day 30, the following factors were measured using both blood and urine samples collected from the participants: Cr (creatinine), GFR (glomerular filtration rate), VBG (venous blood gas), BUN/Cr (blood urea nitrogen to creatinine ratio), U/A (urinalysis), and serum P (serum phosphate). These measurements were assessed for all individuals in both the intervention and control groups. On day 7, Cr and GFR were measured using blood samples obtained from the laboratory. These measurements were also assessed for all individuals in the intervention and control groups.
The study aimed to evaluate the effects and outcomes of the intervention and control groups throughout the study by analyzing these measurements and endpoints. The author, M.M.G., recorded the data without knowledge of grouping information to maintain study masking.
3.4. Statistical Analysis
We used descriptive statistics to summarize the data. Frequencies and percentages were used for categorical variables, while continuous variables were presented as mean ± standard deviation. Furthermore, inferential analyses were conducted using several statistical tests, including the t-test, Analysis of Variance (ANOVA), chi-square test, and repeated measures test. Statistical significance was determined by a P-value of less than 0.05, indicating the presence of significant relationships or differences in the data. All statistical analyses were carried out using IBM SPSS version 27.
4. Results
Of 30 patients in each group, 3 from the control group and 7 from the vitamin E group were lost to follow-up. Consequently, 50 patients were enrolled in this trial, with a mean age of 29.90 ± 7.33. In this study, we included 23 (46%) males with a mean age of 30.13 ± 7.93 and 27 (54%) females with a mean age of 29.74 ± 6.93. There was no significant difference in age between male and female patients (P = 0.854) (Table 1).
| Group | Control | Vitamin E | Statistic/ P-Value | |
|---|---|---|---|---|
| Chi-Square Test | t-Test | |||
| Age | 29.27 ± 6.72 | 30.64 ± 7.08 | 0.487 | |
| Gender | 0.368 | |||
| Male | 13 (48.1%) | 14 (51.9%) | ||
| Female | 14 (60.9%) | 9 (39.1%) | ||
4.1. Kidney Function
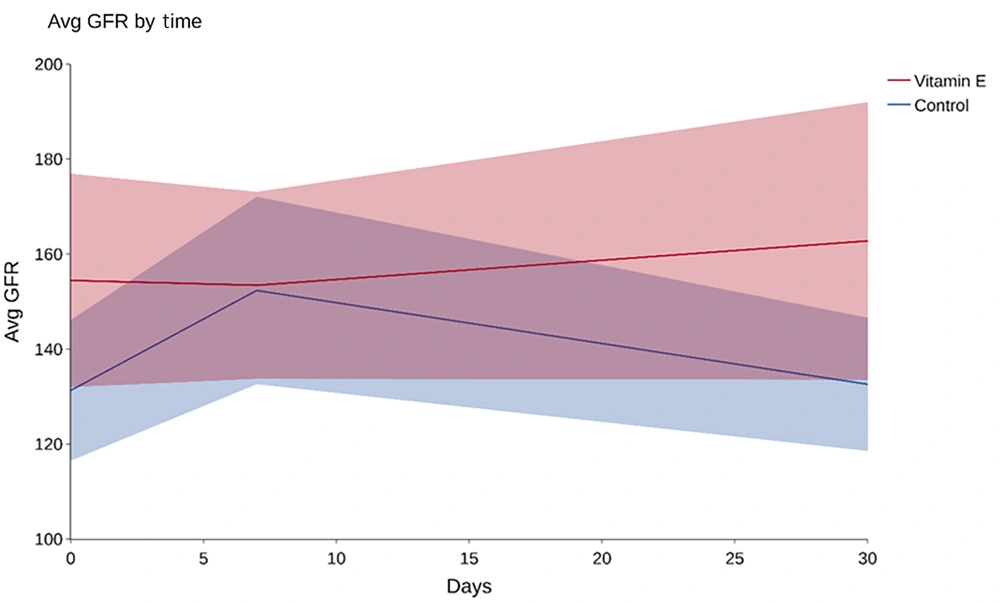
The average GFR was 141 ± 45 at baseline among 50 participants, including 131 ± 37 in the control group and 154 ± 51 in the intervention group. A t-test showed a P-value of 0.074, indicating no significant difference in baseline GFR between the study groups, as depicted in Figure 2.
Average GFR changes from baseline to Day 7 and Day 30 are shown. No significant difference was observed between the control and intervention groups. Table 2 provides additional details.
| Group | Control | Vitamin E | P-Value |
|---|---|---|---|
| GFR baseline | 131.30 ± 37.43 | 154.46 ± 51.95 | 0.074 |
| GFR day 7 | 152.34 ± 49.90 | 153.44 ± 45.53 | 0.936 |
| GFR day 30 | 132.58 ± 35.45 | 162.77 ± 67.60 | 0.063 |
| BUN/Cr baseline | 37.26 ± 21.28 | 32.62 ± 16.85 | 0.403 |
| BUN/Cr day 30 | 34.62 ± 12.63 | 34.53 ± 21.00 | 0.985 |
| Cr day 7 | 0.115 ± 0.23 | 0.003 ± 0.06 | 0.019 |
| Cr day 30 | 0.005 ± 0.19 | 0.006 ± 0.12 | 0.984 |
On Day 7, the overall mean GFR was 152 ± 47, including 152 ± 49 in the control group and 153 ± 45 in the intervention group. A t-test on the GFR’s relation to 7-day vitamin E consumption resulted in a P-value of 0.936, indicating no significant link (Figure 2). By day 30, the mean GFR was 146 ± 54 overall: 132 ± 35 in the control and 162 ± 67 in the intervention group. An analysis showed a P-value of 0.063 regarding the GFR and 30-day vitamin E consumption, again suggesting no significant association (Figure 2 and Table 2).
In this study, the baseline mean BUN/Cr ratio for all participants was 35 ± 19, with 37 ± 21 in the control group and 32 ± 16 in the intervention group. A P-value of 0.403 suggested no significant association between BUN/Cr ratios and the study groups at the baseline.
On day 30, the overall mean BUN/Cr was 34 ± 16, with the control and intervention groups having BUN/Cr of 34 ± 12 and 34 ± 21, respectively. A P-value of 0.985 indicated no significant link between 30-day vitamin E consumption and BUN/Cr.
By day 7, the control group had a mean creatinine increase of 0.115 ± 0.23, while the intervention group showed 0.0026 ± 0.059. With a P-value of 0.019, there was a significant association between vitamin E consumption and lower creatinine increase, suggesting the intervention group benefited more than the control group (Table 2).
By day 30, the control group had a mean creatinine increase of 0.0052 ± 0.19, and the intervention group had 0.0061 ± 0.12 compared to baseline. Examining the link between creatinine increase and vitamin E consumption gave a P-value of 0.984, indicating no significant association on day 30.
4.2. Proximal Tubulopathy
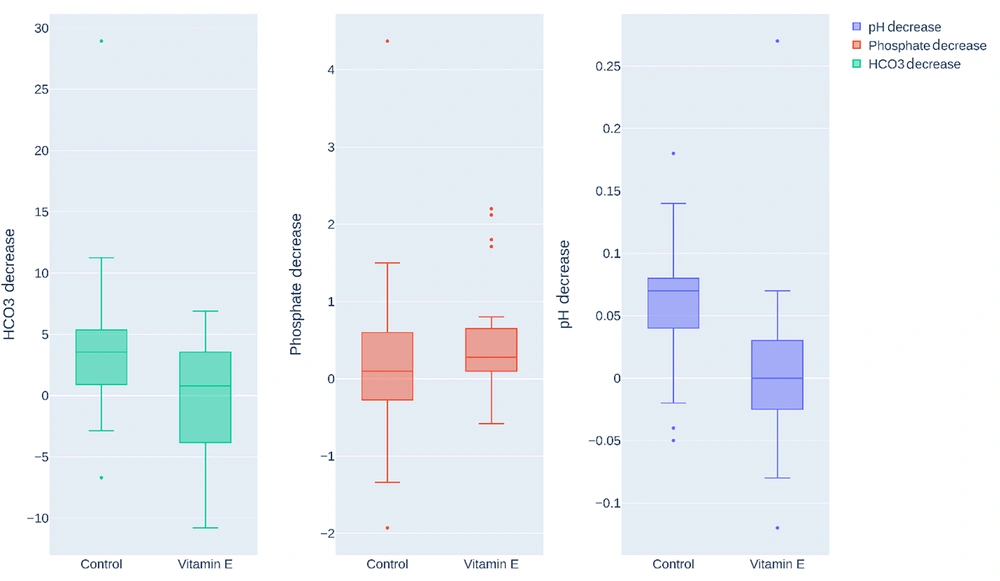
In the fourth week, the control group (n = 27) showed a mean HCO3 reduction of 4.037 ± 6.23, whereas the vitamin E group (n = 23) had an increase of 0.0039 ± 4.49. An independent t-test revealed a P-value of 0.013, denoting a significant association between vitamin E consumption and a lesser reduction in HCO3, as depicted in Figure 3.
Box plots compare reductions in PH, serum phosphate, and bicarbonate (HCO3) levels in the control and vitamin E groups after four weeks. Vitamin E intake was associated with a significantly smaller decrease in HCO3 (P = 0.013) and a lower PH reduction (P = 0.003), indicating potential protective effects. However, no significant difference was observed in serum phosphate reduction (P = 0.391).
In the fourth week, the control group showed a mean serum phosphate reduction of 0.249 ± 1.09, while the vitamin E group showed a decrease of 0.49 ± 0.77. An independent t-test gave a P-value of 0.391, suggesting no significant association between vitamin E consumption and serum phosphate reduction, as shown in Figure 3.
In the fourth week, the control group had a mean pH reduction of 0.0607 ± 0.49, while the vitamin E group showed a decrease of 0.0065 ± 0.49. An independent t-test showed a P-value of 0.003, indicating a significant association between vitamin E consumption and a smaller reduction in PH. Therefore, individuals consuming vitamin E had a notably lesser PH decrease than the control group, suggesting that vitamin E prevents acidosis, as detailed in Table 3.
| Group | Control | Vitamin E | P-Value |
|---|---|---|---|
| HCO3 decrease | 4.037 ± 6.23 | - 0.004 ± 4.50 | 0.013 |
| Phosphate decrease | 0.249 ± 1.09 | 0.49 ± 0.77 | 0.391 |
| PH decrease | 0.061 ± 0.05 | 0.007 ± 0.07 | 0.003 |
a Negative values indicate an increase.
5. Discussion
The primary objective of this study was to explore the preventive effect of vitamin E when combined with standard iron overload therapy in patients with beta-thalassemia major. Specifically, the aim was to assess its potential in preventing Deferasirox-induced acute kidney injury and proximal tubulopathy. None of the 50 participants developed AKI. However, our results indicate that adding vitamin E to standard therapy can benefit kidney function.
Deferasirox is generally well-tolerated but can lead to AKI, especially in high-risk patients with beta-thalassemia major. Also, AKI caused by Deferasirox is a significant factor in hospitalization and mortality in this patient population. Besides, AKI has been associated with higher mortality rates and adverse outcomes. There is a growing trend of nephrotoxic factors leading to an increased risk of AKI in the United States. The occurrence of AKI exposes patients to elevated risks of subsequent cardiovascular events, prolonged hospital stays, progression to end-stage renal disease, all-cause mortality, and escalated acute care expenses. Consequently, mitigating the incidence of AKI is a paramount patient safety objective. By adopting evidence-based preventive strategies, the United States has the potential to avert thousands of AKI cases each year (17).
In an FDA newsletter published on September 18, 2007, 16 suspicious duplicate reports of renal adverse reactions associated with Deferasirox were mentioned, including kidney failure, acute kidney injury, glomerulonephritis, interstitial nephritis, and renal tubulopathy. The onset of AKI occurred 15 days (range 5 - 58 days) after starting treatment with Deferasirox. In 2008, Kidney Safety Advisory Board members explicitly expressed their doubts about Deferasirox being the cause of any reported kidney failure (18).
Previous studies have identified the multifaceted pathogenesis of Deferasirox-induced AKI. A study conducted to evaluate tubular and glomerular function before and after initiating DFX treatment in a pediatric patient population found that GFR decreased by 20% following DFX treatment, and two patients experienced a generalized proximal tubular dysfunction. Therefore, evaluation of kidney function is necessary to prevent the development of chronic kidney disease, which may occur due to long-term damage (8).
Interestingly, in the current study, the average GFR of all patients was within the normal range at the beginning. After one month of evaluation, the average GFR did not significantly differ between the two groups. This result is consistent with a study examining Deferasirox’s effects on GFR in desert rats, where Deferasirox was injected intraperitoneally in rats and showed no reduction in GFR (19).
Studies investigating Deferasirox-induced nephrotoxicity have employed various markers, including serum creatinine (sCr), serum cystatin C, inulin clearance, and tubular dysfunction, to define kidney injury. The reported incidence of kidney toxicity varied widely depending on the method used, ranging from < 10% to 100%. A non-progressive increase in sCr levels (more than a 33% increase on two consecutive occasions) has been observed in about one-third of Deferasirox-treated patients (18). In the present study, serum creatinine was used as an indicator, and the average increase in creatinine during the first week was significantly lower in the intervention group than in the control group. However, using more sensitive markers like cystatin C or inulin clearance could have provided more reliable results.
Insights from studies of other tubular toxicants were considered to investigate the sensitivity of proximal tubular cells to Deferasirox toxicity (20). Proximal tubular cells are specialized for membrane transport and have abundant mitochondria, providing the required energy for transport processes and aiding in the excretion and retrieval of substances. Drugs excreted through tubular secretion quickly reach proximal tubular cells, leading to higher intracellular drug concentrations than other cell types. Additionally, mitochondrial toxicity can impair tubular cell function and survival, potentially leading to kidney failure (21).
The lipophilicity of Deferasirox allows it to enter many cells easily (22). Deferasirox is 99% bound to proteins, mainly albumin. This high level of protein binding is consistent with tubular secretion compatible with proximal tubular kidney toxicity (18). This hypothesis is supported by clinical studies conducted with Deferasirox in rats, where high doses of Deferasirox were associated with vacuolization of proximal tubular epithelium, ultimately leading to nephrotoxicity without compromising glomerular function. This Deferasirox-induced side effect appears to be related to apoptotic events and possibly linked to mitochondrial dysfunction and reduced ATP levels (19).
In a case study, a patient undergoing Deferasirox treatment developed hyperchloremic metabolic acidosis, which improved after stopping the iron chelator. This type of acidosis is primarily associated with kidney issues in individuals without diarrhea or kidney failure, as observed in the case. It is hypothesized that Deferasirox’s lipophilic nature enables it to enter cells and bind to intracellular iron, essential for ATP production, resulting in a critical cellular fuel deficiency (23).
A study reported a case of hyperchloremic metabolic acidosis in a patient undergoing Deferasirox treatment, which resolved after discontinuing the chelator and administering oral iron. This type of acidosis is mostly related to kidney issues in individuals without diarrhea or renal insufficiency, similar to the discussed case. Deferasirox’s high-fat solubility has been suggested to facilitate its entry into cells at elevated levels and binding to intracellular iron, which is essential for cellular energy production. This can result in a severe deficiency of cellular fuel. However, our study showed that the average PH and HCO3 levels were within the normal range in both groups at the beginning and end of the study (23).
Several clinical trials have explored the antioxidant properties of vitamin E and its protective role against oxidative stress. However, the debate over its preventive effect on AKI remains. Our findings, based on a study with 29 CKD patients treated with contrast material, did not demonstrate significant benefits of vitamin E in preventing Contrast-induced Acute Kidney Injury (CIAKI) due to the absence of AKI in the study groups (24).
Recently, the focus on vitamin E has significantly increased due to its antioxidant properties, particularly alpha-tocopherol, the main compound of interest. Its intestinal absorption, hepatic transport, and cellular uptake have been extensively studied and described (25). In our study, we administered a daily dose of 400 IU of vitamin E, but we could not assess its preventive effect on Deferasirox-induced AKI due to the absence of AKI in any of the groups. However, another randomized controlled trial on 103 Thai CKD patients who underwent selective coronary angiography showed that oral intake of 525 international units of alpha-tocopherol led to a significant 17.2% reduction in CIAKI (5.9% in the intervention group and 23.1% in the placebo group) (26). This finding suggests that the effectiveness of vitamin E may depend on dosage and administration methods. Injectable administration may be more effective, as oral consumption has uncertainties related to absorption and first-pass effects, influenced by factors like pancreatic lipase secretion and chylomicron formation (27).
Vitamin E has shown potential in preventing renal characteristics in certain types of diabetic kidney disease (15). Our study found promising results, with one week of vitamin E significantly preventing serum creatinine elevation. However, the protective effect was not significant after one month of consumption. A previous report in 2013 highlighted renal impairment caused by Deferasirox in patients with β-thalassemia major, where one year of treatment led to a considerable increase in serum creatinine levels and a decrease in creatinine clearance, especially in patients above 18 years of age compared to younger patients (18).
This report indicates that although many patients taking Deferasirox may not develop AKI, Deferasirox can increase the average serum creatinine level during chronic use. Therefore, according to our findings, vitamin E consumption has been able to reduce the average increase in serum creatinine in thalassemia patients taking Deferasirox, and we speculate that long-term consumption and higher doses of vitamin E may prevent the long-term increase in serum creatinine levels, which requires a study with larger sample size and longer duration.
Our study observed that the PH and HCO3 levels in both groups remained within the normal range throughout the study. Vitamin E consumption did not affect serum phosphorus levels, which remained normal in both groups. However, vitamin E significantly prevented the decrease in HCO3 levels and was associated with a lower decrease in PH, suggesting a potential preventive effect against acidosis. Urine sample analyses for various markers were normal in both groups at the beginning and end of the study, making it difficult to evaluate the relationship between vitamin E and tubulopathy prevention.
Regarding kidney function, consuming vitamin E for one week effectively prevented an increase in serum creatinine levels, while its preventive effect was insignificant after one month of consumption.
5.1. Limitations
In the trial, 60 participants were initially enrolled, but the analyzed sample was reduced to 50 due to losses to follow-up. A follow-up period of just 30 days was utilized, and the short duration might not have been adequate to note the long-term impacts of vitamin E. Individuals with certain medical conditions and histories were excluded, possibly narrowing the study’s applicability. Emphasis was given to specific kidney function markers, potentially overlooking a comprehensive view of kidney health. The study’s generalizability might be compromised as it was conducted in a single Iranian hospital. The brief one-month intervention, the fact that only participants were blinded to their group assignments, and the absence of a placebo in the control group might have influenced the study’s conclusions.
5.2. Conclusions
This study showed that the daily consumption of 400 IU of vitamin E in thalassemia patients taking a daily dose of 20 mg/kg of Nanojade iron chelator tablets could prevent increased creatinine in the first week of consumption. It can also avoid acidosis by decreasing HCO3 and PH, but it does not affect GFR, serum phosphorus, or BUN. Additionally, since none of the individuals in our study developed AKI or proximal tubulopathy, their evaluation and relationship with the consumption of vitamin E could not be assessed.