1. Background
Nephrotic syndrome (NS) is one of the most widely recognized kidney diseases in children (1). It is characterized by the leakage of protein from the blood into the urine through the damaged glomerulus, resulting in proteinuria, hypoalbuminemia, generalized edema, and hyperlipidemia (1, 2). Apart from congenital NS, there are various etiologies, including glomerular disease, vasculitis, infections, toxins, malignancies, and genetic changes (1, 2). Approximately 80% to 90% of children with idiopathic NS (INS) respond to steroid treatment (steroid-sensitive nephrotic syndrome, SSNS), while the remainder do not respond to steroids (steroid-resistant nephrotic syndrome, SRNS) (2, 3). Depending on the pathology and severity of the disease, there is a risk of thrombosis in children with NS, leading to greater morbidity and mortality in 20-30% of adults and around 27% of children (4).
The underlying mechanisms of thromboembolism in INS are multifactorial (4, 5). Disorders of the coagulation system appear to play an important role in venous thrombotic events, and platelet counts have a significant role in the thrombus-promoting state of INS (5). Platelets are the smallest but highly reactive blood cells, primarily involved in the fibrosis process and maintenance of normal hemostasis (3, 6). Larger platelets are more metabolically and enzymatically active and release more thromboxane A2, thrombomodulin, and adhesion molecules (6). Mean Platelet Volume (MPV) is a practical and predictive biomarker for cardiovascular disease (CVD) in patients with renal failure (7)). Currently, the severity of coronary artery disease, vascular changes in atherosclerosis, and platelet aggregation have been shown to be independent of MPV (8). Some studies have suggested a link between stroke risk and MPV, but not a link between stroke subtypes, infarct severity, and functional recovery (9). Average platelet volume can provide important information regarding the course and prognosis of pathological diseases such as respiratory diseases, Crohn's disease, rheumatoid arthritis, juvenile systemic lupus erythematosus, and neoplastic diseases (10). The mechanism of increased platelet counts in NS has not been studied and may be multifactorial, possibly due to hypoalbuminemia and nephrotic hypercholesterolemia syndrome, resulting from changes in plasma levels of platelet-interfering proteins and lipid changes due to the disease (11). Platelets are also considered an acute response in various inflammatory processes, and MPV is not only a method of platelet activation but also a simple, cheap, and easy method to determine disease prognosis and steroid resistance (12). Careful follow-up is important in patients who develop focal segmental glomerulosclerosis, especially those with low MPV and thrombocytosis (13).
Considering the above literature and the importance of platelet count and platelet size in the diagnosis of many diseases such as nephrotic syndrome, and also considering their clinical utility, evaluating their possible role in nephrotic syndrome is necessary.
2. Objectives
This study aimed to investigate changes in platelet count and mean platelet volume in pediatric nephrotic syndrome.
3. Methods
3.1. Study Type and Place
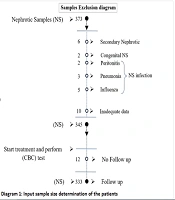
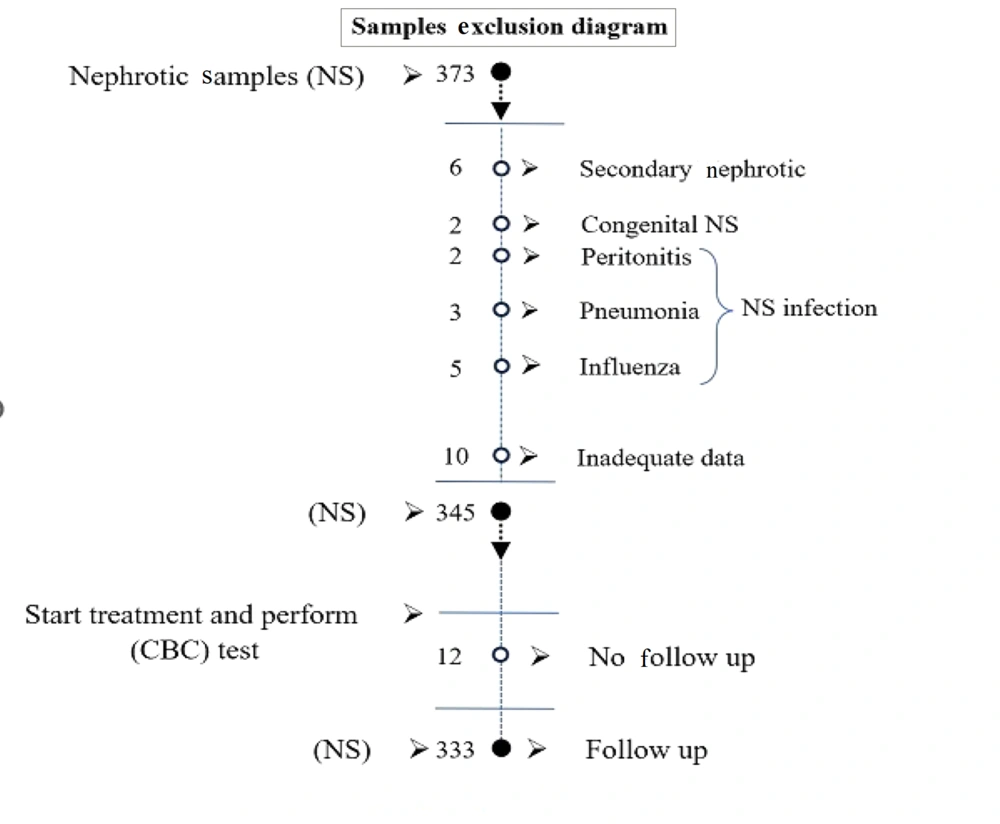
Children under the age of eighteen who were admitted to a tertiary care hospital in Southeast Iran over the course of a decade, until 2021, with idiopathic NS (INS) were the subjects of this hospital-based case-control study. A total of 373 children diagnosed with NS during this time were admitted to the Pediatric Nephrology Ward of the Ali Ibne Abitalib Hospital, which is affiliated with Iran's Zahedan University of Medical Sciences. After considering the exclusion criteria, 333 children were included in the study. Figure 1 depicts the sample size attrition due to the exclusion criteria. Additionally, 156 children who were sent to the hospital for a standard examination were included in the healthy control group.
3.2. Study Considerations
The inclusion criteria for patients with NS were generalized edema, massive proteinuria (> 40 mg/kg/day, or urine protein/Cr > 2.0 mg/mg), hypoalbuminemia (serum albumin < 2.5 g/day), and hyperlipidemia. Healthy children who were sent to a children's hospital for standard checkups were included in the control group. We split our patients into two groups. Steroid-sensitive nephrotic syndrome (SSNS) was typified by 0 to trace urine protein for three consecutive days following daily administration of prednisolone at a dose of 2 mg/kg/day for four weeks. Patients who failed to meet these criteria were classified as steroid-resistant.
Proteinuria was assessed by 24-hour urine protein excretion and level, and a level of 40 mg/m2/h or a P/Cr ratio higher than 2 in random urine. Remission was considered when proteinuria was 4 mg/m2/h and albumin levels were within normal limits. To assess MPV, approximately 2 mL of citrated blood was collected from patients and controls and analyzed using the same direct impedance method with Sysmex Colter KX-21N and Sysmex 300-XP devices. Platelet histograms were obtained from large-angle light scattering measurements. Two mL of venous blood in a smooth tube was left in an incubator to clot, and the separated serum was centrifuged. Biochemical parameters such as serum albumin, total cholesterol, urea, and creatinine were measured using spectrophotometry on an Abbott Architect C-8000 instrument (Abbott Diagnostics, Santa Clara, CA).
3.3. Exclusion Criteria
Children with secondary NS, congenital nephrosis, inadequate data in the profiles, those dropping out during follow-up, urinary tract infections, peritonitis, and patients older than 18 years were excluded from the study.
3.4. Ethical Approval
Informed consent was obtained from parents after the study protocol explanation. Ethical approval was obtained from the ethical committee of the research deputy, Zahedan University (IR.ZAUMS.REC.1399.283).
3.5. Collected Information
Demographic characteristics of the patients (age, sex, weight, blood pressure), type of nephrotic syndrome, and laboratory parameters, including hemoglobin, hematocrit, white blood cell count, platelet counts, MPV, blood urea nitrogen, creatinine, electrolytes, albumin, and the amount of proteinuria in active and remission phases, were evaluated. Baseline laboratory parameters at diagnosis of nephrotic syndrome and during the remission phase were compared between the SSNS and SRNS groups.
3.6. Statistical Analysis
Data analysis was performed with SPSS 20 (SPSS, Inc., Chicago, IL). The normality of data distribution was assessed using the Kolmogorov-Smirnov test. All descriptive data had a free distribution. Therefore, the results for the quantitative variables were reported as mean ± standard deviation (SD), and the ordinal qualitative variables were reported as frequencies and percentages. The Kruskal–Wallis test by ranks and the Mann–Whitney U test were used to compare the quantitative variables. For the association between the variables, Pearson correlation was applied. The levels of P-value and confidence coefficient were considered 0.05 and 95%, respectively.
4. Results
There were observed changes in age, weight, PLT count, and MPV among the three participant groups, as demonstrated in Table 1. The mean values for PLT were 399.53 ± 128.70, 467.97 ± 130.75, and 336.05 ± 98.35 in the responder, resistance, and control groups, respectively. The distribution between groups showed a significant result (chi-square = 60.491, P < 0.05). In terms of the MPV values, the responder group had an average of 7.99 ± 1.58, the resistance group had an average of 7.81 ± 0.96, and the control group had an average of 9.33 ± 1.1. The distribution between groups showed a significant difference, with a chi-square value of 124.806 and a p-value of less than 0.05.
| Variables and Groups | N | Mean Rank | Chi-square | P-Value |
|---|---|---|---|---|
| Age | 0.662 | 0.718 | ||
| Responders | 266 (5.42 ± 2.94) | 240.47 | ||
| Resistant | 67 (5.79 ± 3.66) | 246.91 | ||
| Control | 156 (5.54 ± 2.66) | 251.9 | ||
| Total | 489 (5.51 ± 2.96) | - | ||
| Weight | 5.02 | 0.081 | ||
| Responders | 266 (18.89 ± 8.45) | 242.42 | ||
| Resistant | 67 (21.05 ± 9.62) | 279.76 | ||
| Control | 156 (17.86 ± 6.22) | 234.46 | ||
| Total | 489 (18.86 ± 8.03) | - | ||
| PLT count | 60.491 | < 0.001 | ||
| Responders | 263 (399.53 ± 128.7) | |||
| Resistant | 66(467.97 ± 30.75) | 257.65 | ||
| Control | 156 (336.05 ± 98.35) | 332.13 | ||
| Total | 485 (388.42 ± 27.18) | 180.6 | ||
| MPV | 124.806 | < 0.001 | ||
| Responders | 264 (7.99 ± 1.58) | 201 | ||
| Resistant | 67 (7.81 ± 0.96) | 172 | ||
| Control | 156 (9.33 ± 1.1) | 346 | ||
| Total | 487 (7.90 ± 1.01) | - |
aValues are expressed as mean ± SD.
Table 2 showed that PLT counts had different values in pairs of responders-resistant, responders-controls, and resistant-controls. Similar trends were observed for MPV, except in the pair of responders-resistant.
| Variables and Groups | Groups | P-Value |
|---|---|---|
| PLT count | ||
| Responders | Resistant | < 0.001 |
| Control | < 0.001 | |
| Resistant | Control | < 0.001 |
| MPV | ||
| Responders | Resistant | 0.098 |
| Control | < 0.001 | |
| Resistant | Control | < 0.001 |
Table 3 revealed that for all patients, the average PLT levels were 413.26 ± 131.81 and 331.26 ± 102.28 in the active phase and remission, respectively, which was highly significant (P < 0.001). In individuals experiencing the active phase, there was a notable difference in the means of MPV, with an average of 8.28 ± 1.22. Those in remission had a slightly lower mean MPV of 7.96 ± 1.04, which was also statistically significant (P < 0.001).
| Patients | Variables | Groups | Mean ± SD | Minimum | Maximum | T-Value | P-Value |
|---|---|---|---|---|---|---|---|
| All patients | PLT count | Active phase | 413.26 ± 131.80 | 110 | 935 | -10.809 | < 0.001 |
| Remission | 331.26 ± 102.28 | 117 | 750 | ||||
| MPV | Active phase | 7.96 ± 1.04 | 5.3 | 11.2 | -7.153 | < 0.001 | |
| Remission | 8.28 ± 1.22 | 5 | 12 | ||||
| Responders | PLT count | Active phase | 399.53 ± 128.70 | 110 | 864 | -8.366 | < 0.001 |
| Remission | 332.52 ± 104.06 | 117 | 750 | ||||
| MPV | Active phase | 8.00 ± 1.06 | 5.3 | 11.2 | -5.789 | < 0.001 | |
| Remission | 8.27 ± 1.21 | 5.7 | 12 | ||||
| Resistant | PLT count | Active phase | 467.97 ± 130.75 | 135 | 935 | -6.685 | < 0.001 |
| Remission | 326.24 ± 95.44 | 127 | 654 | ||||
| MPV | Active phase | 7.81 ± 0.96 | 5.9 | 11 | -4.32 | < 0.001 | |
| Remission | 8.31 ± 1.26 | 5 | 11 |
For responder patients, the average PLT levels were 399.53 ± 128.70 in the active phase and 332.26 ± 1040.06 in remission, showing a significant difference (P < 0.001). The mean MPV values were 8.00 ± 1.06 in the active phase and 8.27 ± 1.21 in remission, with this difference being statistically significant (P < 0.001).
In the resistant group, the mean PLT levels were significant (P < 0.001) between the active phase (467.97 ± 130.75) and remission (326.24 ± 95.44). The mean difference in MPV was also significant (P < 0.001) between the active phase (7.81 ± 0.96) and remission (8.31 ± 1.26).
Changes in PLT count and MPV were observed before and after the treatments, as shown in Table 4. It was found that the responder patients had an average MPV change of 0.27 ± 0.97, while the resistant patients had an average MPV change of 0.52 ± 1.14, indicating a higher value in the resistant patients (P = 0.016). The levels of PLT changes in responder and resistant patients were 0.27 ± 0.97 and 0.52 ± 1.14, respectively, indicating elevated values in the resistant patients (P = 0.016). Patients who resisted treatment exhibited significantly higher PLT changes before and after treatment (141.73 ± 98.39) compared to responders (67.01 ± 128.00), with a significant difference (P < 0.001).
| Before-After Treatment and Groups | N | Means ± SD | Mean Rank | P-Value |
| MPV | 0.016 | |||
| Responders | 263 | 0.27 ± 0.97 | 158.67 | |
| Resistant | 66 | 0.52 ± 1.14 | 190.23 | |
| PLT count | < 0.001 | |||
| Responders | 263 | 67.01 ± 128.00 | 151.49 | |
| Resistant | 66 | 141.73 ± 98.39 | 218.85 |
According to the analysis findings, there was no substantial correlation (with a correlation coefficient of r > 0.05) between PLT and MPV, and cholesterol, albumin, and protein/creatinine ratio in the patients (Table 5).
| Variables and Statistics | CHL | ALB | Pro/Cre Ratio |
|---|---|---|---|
| PLT count | |||
| r | 0.020 | -0.062 | 0.025 |
| P | 0.728 | 0.269 | 0.654 |
| MPV | |||
| r | 0.044 | -0.032 | 0.003 |
| P | 0.441 | 0.566 | 0.961 |
5. Discussion
The study concluded that PLT counts and MPV scores were different in children with INS compared to controls. These differences showed higher PLT counts in resistant patients, followed by responders and then controls. However, the MPV value was highest in the control group, followed by steroid-responsive patients, and then steroid-resistant patients. Platelets play an important role in the development and complications of atherosclerosis. As a result, a higher PLT count is likely to increase granules and high metabolic and enzymatic activity, causing thrombi (14).
Gulleroglu et al. (15) conducted a study to examine the significance of changes in mean platelet volume (MPV) during periods of activity and remission in children diagnosed with idiopathic nephrotic syndrome (INS). In his study, a comparison was made between 55 children diagnosed with INS and 29 healthy children. The results revealed that the patient group had a significantly higher number of PLTs compared to the control group.
In the study by Yousefichaijan et al. (16), the children were divided into two groups, and it was discovered that there was a significant difference in the number of PLTs among children with a history of INS and those with frequent relapses, as well as NS-dependent and steroid-resistant children. Additionally, it was discovered that among the children who positively reacted to steroids, approximately 92% displayed normal PLT counts, 8% had counts above the normal range, and none showed counts below the normal level. On the other hand, within the other group, only around 26% exhibited normal counts. In our investigation, it was discovered that 70% had PLTs that were higher than the usual levels, while 4% had PLTs that were lower.
Regarding MPV, it was determined that there was insufficient disparity among the groups. In the study conducted by Kucuk et al. (17), the researchers aimed to assess MPV's value in determining disease activity and symptoms associated with polyangiitis granulomatosis (GPA), compared to a control group. Patients with GPA had a notably lower MPV compared to other groups. The mean platelet volume (MPV) showed a negative correlation with both the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in the patient group. In various inflammatory processes, platelets play a critical role as part of an acute response.
In individuals suffering from urinary tract infections, platelets (PLT) play a crucial role in the body's defense system. They aid in the host's defense by encouraging phagocytosis and generating cytotoxic free radicals alongside oxidative molecules. Hence, the PLT count serves as a speedy and supplementary diagnostic test for children afflicted with urinary tract infections (18).
In their study, Kocyigit et al. (19) set out to investigate the significance of platelet activation in determining the prognosis of NS. They evaluated 156 treated primary NS patients for their MPV and subsequently monitored them for a period of one year. The patients were categorized into three groups: Complete response, partial response, and resistance. The researchers discovered a noteworthy rise in MPV levels within the resistance group compared to the other two groups.
A study conducted by Nickavar et al. (20) on children evaluated the platelet count in INS. The researchers found that patients who were resistant to steroids had noticeably higher platelet counts compared to patients who were sensitive to steroids. The study revealed that the area under the ROC curve, along with low mean platelet volume (MPV) and a wide platelet distribution, displayed a reasonably satisfactory correlation with the response to steroid treatment.
According to reports, the PLT count remained elevated during remission following the initiation of corticosteroid treatment and immediately after discontinuing corticosteroid treatment (11). This finding contrasts with the study conducted by Mittal et al. (21) in India. In their study, Gulleroglu et al. (15) discovered a notable, yet adverse, correlation between the average mean platelet volume (MPV) and the average platelet count. There was a notable positive correlation observed between the average platelet count and the average levels of total cholesterol and triglycerides. In the resistance group, there was a considerable increase in MPV levels, while only LDL cholesterol witnessed a significant decrease. Furthermore, it has been shown that MPV changes were significantly correlated with proteinuria, CRP, and albumin. A significant prediction regarding the alteration of proteinuria was made based on the statistical analysis of albumin, total cholesterol, and CRP.
Several studies have demonstrated that an elevated MPV level is linked to several cardiovascular risk factors, including hypertension, diabetes, dyslipidemia, insulin resistance, and metabolic syndrome (22). Prior to initiating treatment, a decrease in mean platelet volume (MPV) was observed in children with NS (23). It has been suggested that hypoalbuminemia may play a role in the increase of PLTs. Plasma albumin levels exhibited an inverse correlation with PLT count (23). In this particular matter, our study did not find any connection between PLT and MPV levels with cholesterol, albumin, and protein/creatinine ratios. Kocyigit et al. (19) showed a significant increase in MPV levels, while only LDL cholesterol levels showed a significant decrease. The associations between the variations of MPV and proteinuria, CRP, and albumin were found to be noteworthy.
In their study, Gulleroglu et al. (15) did not find a correlation between platelet count and hypoalbuminemia. However, they did observe a notable relationship between mean platelet counts and average total cholesterol and triglyceride levels. Furthermore, a noteworthy inverse correlation was discovered between the average platelet count and MPV. Additionally, the PLT count was found to have no correlation with cholesterol levels in NS patients, as reported in a previous study (23).
In a study conducted by Odimegwu et al. (24), the aim was to evaluate the haemostatic profile of children with NS. It was determined that approximately 15% of the children with NS exhibited thrombocytosis. The median platelet count in the children with the new disease was found to be 432,000, significantly higher than that of the control group. The results of the study mentioned appear to show some degree of comparability with our own study. One limitation of the current study was the exclusion of patients who developed microbial infections during the treatment. Regrettably, we failed to take into account the potential impact of medication on the MPV measurement.
5.1. Conclusions
According to the study, the PLT count was highest in steroid-resistant patients, while MPV was observed to be lower in these patients compared to both the steroid-responsive and control groups. Determining the prognosis and steroid resistance in nephrotic syndrome patients can be made easier and more affordable by utilizing mean platelet volume as a simple method. If patients have low MPV accompanied by thrombocytosis, their response to steroid treatment may be a cause for concern, and they will require close follow-up.