1. Introduction
Renal artery stenosis (RAS) is the decrease in the internal diameter of one or both renal arteries. It is the major cause of hypertension (1). Regarding gender prevalence, RAS due to fibromuscular dysplasia is more common in women (2). In an autopsy study, atherosclerotic RAS was observed in 27% of patients who were over 50 years old. If the patient had a history of high diastolic blood pressure, this number increased to 53% (3).
The main causes of RAS are atherosclerosis and fibromuscular dysplasia (FMD), with a mean frequency of 75% and 20%, respectively. Other causes of RAS are vasculitis (especially polyarthritis nodosa), neurofibromatosis type 1, thromboembolic disease, arterial dissection, and infrarenal aortic aneurysm (1).
Here, we present a 66-year-old man who was referred to the internal medicine department of Shahid Beheshti Hospital, Qom City, with the chief complaint of abrupt bilateral lower limbs and scrotal edema. After further investigation, we found that the patient had a 99% stenosis in his left renal artery.
2. Case Presentation
In March 2019, a 66-year-old man with a history of mild chronic kidney disease (CKD), coronary artery disease (CAD), benign prostatic hyperplasia (BPH), and hypertension (HTN), was referred from the emergency unit to the Internal Medicine Department of Shahid Beheshti Hospital, Qom City, with the chief complaint of abrupt bilateral lower limbs and scrotal edema. It was a 4+ pitting edema, and the patient mentioned that he had facial edema at first, which was resolved over time. The patient also had function class III dyspnea and sleepiness. The past medical history of the patient included a mild CKD (with serum Cr levels <2.5 mg/dL and a mild decrease in the size of the right kidney) and a history of cardiac angiography in 2011. Four months ago, he had also visited an ophthalmology clinic due to blurred vision and was diagnosed with uncontrolled blood pressure. Other associated symptoms on admission were itching, a decrease in urine volume for five days, dysuria, dribbling, hesitancy, weakness, and anorexia. Symptoms, such as fever, chills, testicular pain, abdominal pain, cough, phlegm secretion, and sore throat, had not been mentioned. It should be noted that the patient did not have diabetes and did not present a history of tobacco or alcohol consumption. Drug history included sodium bicarbonate, calcitriol 0.25 mg, folic acid 5 mg, prazosin 5 mg, finasteride 5 mg, calcium supplement 500 mg, aspirin 80 mg, valsartan 40 mg, carvedilol 6.25 mg, pantoprazole 40 mg, tamsulosin 0.4 mg, amlodipine 5 mg, and spironolactone 25 mg, which were used either daily or twice a day.
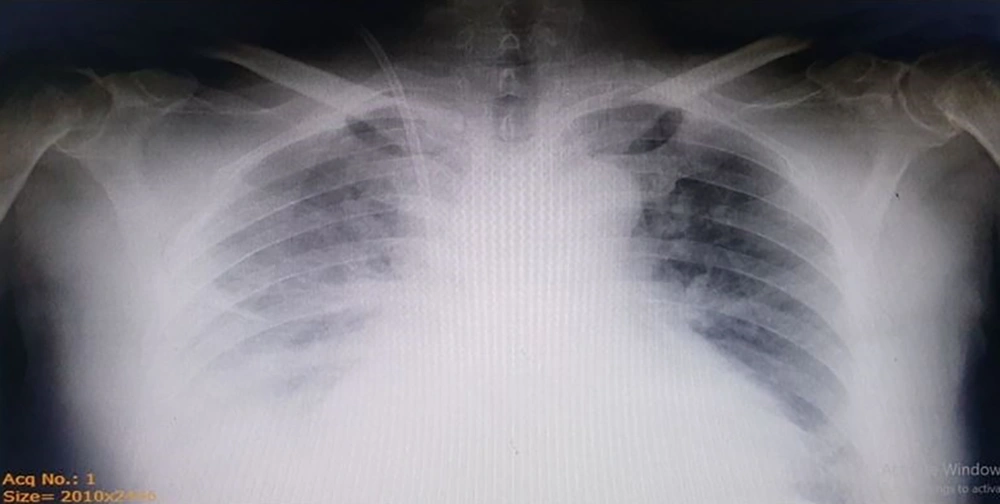
The vital signs included blood pressure of 125/75 mmHg, pulse rate of 80/min, respiratory rate of 19/min, temperature of 37°C, and SpO2 of 98% at the time of admission. The patient was oriented to time and place and was not ill, toxic, or icteric. In physical examination, scratches caused by skin itching were seen. Normal cardiac sounds were heard without murmurs. Crackles in the bases of both lungs were heard in auscultation (Figure 1).
Initial laboratory test results were as follows: Hb: 8.5 g/dL, WBC: 7400 µL, PLT: 162000 µL, Na: 135 mmol/L, K: 5 mmol/L, BS: 209 mg/dL, urea: 249 mg/dL, and Cr: 9.8 mg/dL. Urine analysis also detected sugar 1+, protein 2+, trace blood, white blood cells (4 - 6/HPF), red blood cells (6 - 8/HPF), epithelial cells (10 - 15/HPF), and Ca-oxalate crystals. Urine culture was negative. A Foley catheter was inserted for the patient, and 24-hour urine protein was detected to be less than 400 mg.
In addition, an echocardiography was performed because of the history of CAD. It showed that the patient had minimal pericardial effusion. It was estimated that the left ventricle had a 55% ejection fraction. Pulmonary arterial pressure was also 25 mmHg. An ultrasound of the abdomen and pelvis was also done, and we found that the right kidney of the patient was smaller than the normal size. The left kidney also showed increased cortical echogenicity and multiple areas of calcification. Due to the developed acute kidney injury (AKI), a Shaldon catheter placement surgery was quickly performed, and then the patient underwent three emergency hemodialysis sessions on days 1, 4, and 5.
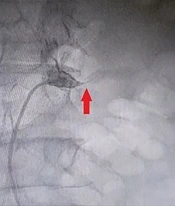
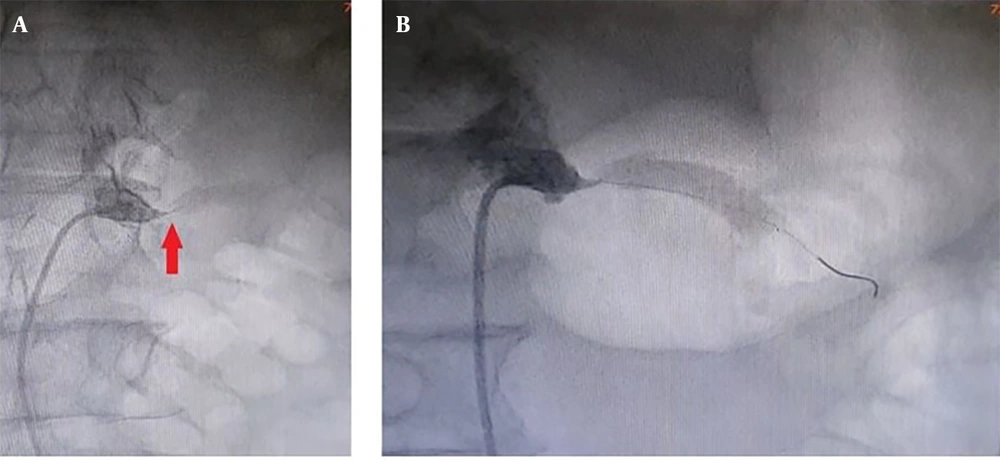
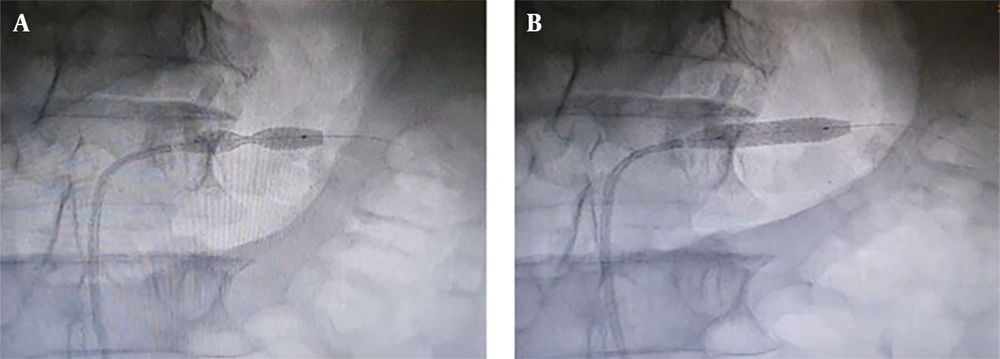
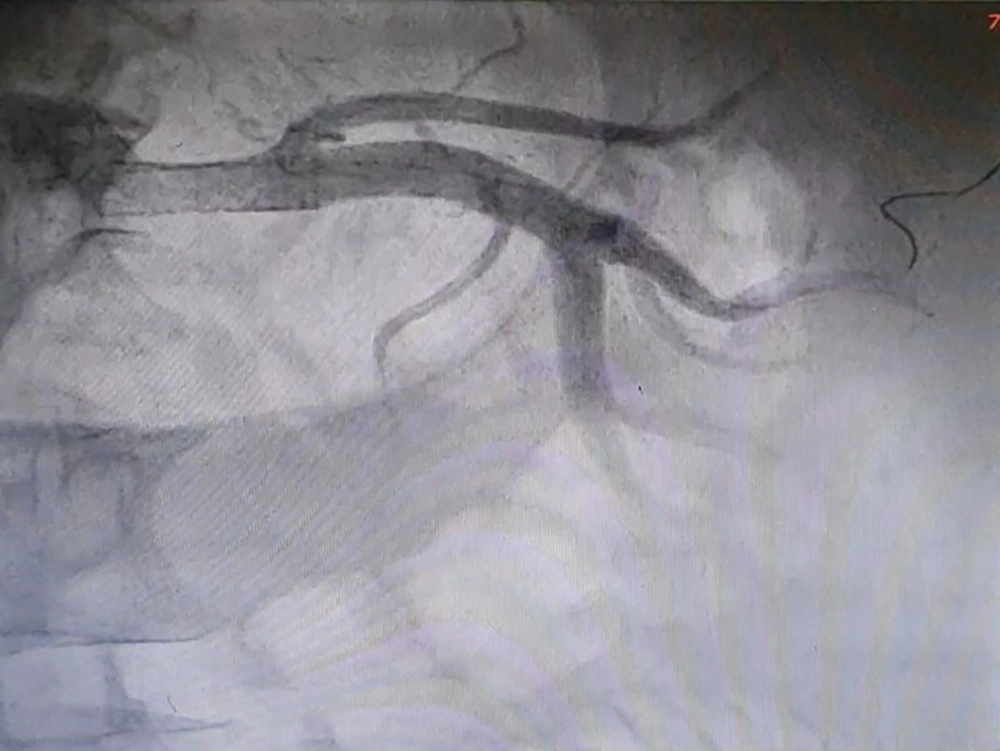
Considering the fact that the patient had a mild chronic decrease in kidney function, a sudden increase in levels of serum Cr and urea was not attributable to other common causes, and the abrupt onset of generalized edema, our suspicion went towards AKI caused by RAS. Therefore, we subjected the patient to an angiography (on the fifth day of hospitalization), which revealed that the left renal artery has 99% stenosis (Figure 2). Therefore, on the same day, a percutaneous Angioplasty with stenting on the left renal artery was performed (using a hippocampus™ 6.5×20 mm stent) to revascularize the left kidney (Figures 3 and 4).
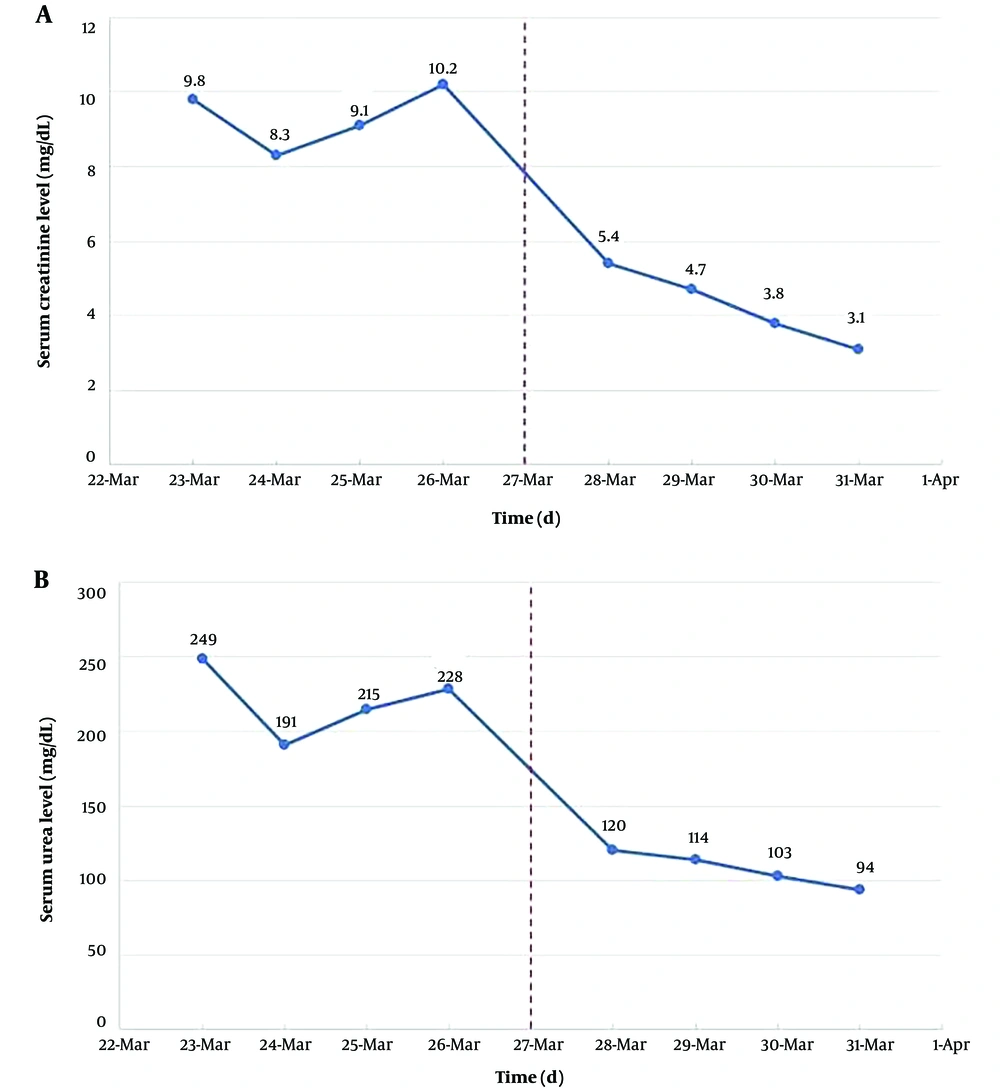
In the next day's laboratory tests, the levels of serum Cr and urea decreased significantly, and this decrease continued in the following days as well (Figure 5). After angioplasty, the patient had a urine output of nearly 8 liters in 24 hours, and his clinical symptoms gradually improved. Finally, after 8 days of hospitalization, the patient was discharged with serum Cr of 3.1 mg/dL, urea of 94 mg/dL, and relatively good general condition.
During ambulatory follow-up from April 2019 to March 2023, the blood pressure of the patient was under control without any hypertension crisis. The latest laboratory tests (March 2023) showed serum Cr was 1.7 mg/dL and urea was 71 mg/dL, indicating the patient had an estimated glomerular filtration rate (eGFR) of about 50 mL/min/1.73m2 and satisfactory kidney function. Currently, the patient receives these medications: Carvedilol 6.25 mg twice a day (BID), prazosin 5 mg BID, Atorvastatin 20 mg BID, Amlodipine 5 mg daily, valsartan 80 mg daily, ASA 80 mg daily, folic acid 5 mg daily.
3. Discussion
Renal artery stenosis (RAS) is a decrease in the internal diameter of one or both renal arteries. It is a major cause of hypertension (1). In our case, blood pressure was 125/75 mmHg at the time of admission. This probably was because of the consumption of antihypertensive drugs by the patient, such as prazosin, valsartan, carvedilol, amlodipine, and spironolactone. Although the patient’s blood pressure fluctuated to some extent during hospitalization, these fluctuations were not significant.
Various pathophysiological mechanisms cause RAS symptoms. These mechanisms include 1-disturbances in the endothelin, 2-kinin-kallikrein system, 3-sympathetic nervous system, 4-angiotensin II, and the renin-angiotensin-aldosterone system (RAAS) (4). Components of the kinin-kallikrein system (KKS) are a group of plasma proteins. These proteins can lead to the induction of genes and the activation of biomolecules that are involved in the molecular mechanisms of vasodilation, blood coagulation, and fibrinolysis (5). The balance between the vasopressor and vasodepressor maintains blood pressure in the normal range. Kinins are a kind of vasodepressor hormone; thus, any paucity in the production of these hormones or damage to their receptor can cause hypertension (6).
The RAAS causes the production of the peptide hormone angiotensin II, and as a result of the production of this protein, aldosterone is produced as well. Both hormones affect the body’s blood pressure and fluid homeostasis control (7). The RAS decreases the volume of blood that enters the kidneys. The juxtaglomerular cells will detect a decrease in blood volume. After these events, the juxtaglomerular activates the RAAS system. This system increases the circulating blood volume and arteriolar tone. These events lead to hypertension (8).
In the past, most studies were performed postmortem or sampled from patients undergoing angiography. For example, in one study, out of 14,152 patients who underwent angiography, 5.1% had less than 50% stenosis in one, or both renal vessels, and 6.3% had a significant RAS (9). In another study, 6.8% of elderly patients enrolled in the study had ≥60% stenosis in one or both renal arteries (10). The classification provided by the American Heart Association (AHA) for patients with RAS is as follows: Grade I: RAS is present with no clinical manifestations (normotensive with normal renal function). Grade II: RAS is present, plus medically controlled hypertension and normal renal function. Grade III: RAS is present, plus evidence of abnormal renal function, medically refractory hypertension, or evidence of volume overload (11). In this case, stenosis was 99% in the left renal artery, and the patient was admitted to the hospital with acute renal dysfunction and severe generalized edema, which means it was a grade III RAS.
The RAS may be unilateral or bilateral. Even the stenosis can occur in the abdominal aorta. On the other hand, a patient with RAS may have a single kidney, and each of these situations requires different approaches. Our patient had a mild CKD for a couple of years, and his right kidney was smaller in size than the left one in ultrasound. However, other than some episodes of hypertension crisis (which had not been well documented), the patient had a steady situation in terms of biomarkers of kidney function (serum urea and Cr and eGFR), and the hypertension was generally under control with oral medications. After the patient experienced abrupt severe generalized edema and a decrease in urinary output, the consultant nephrologist assumed that the right kidney could have been actually non-functional, so unilateral RAS in the left renal artery became a suspicion.
Nearly 10% of all RAS cases occur due to renal FMD. This type usually happens in females <50 years of age and mostly affects the right renal artery rather than the left one. About 90% of RAS cases are atherosclerotic, which generally happens in patients >50 years old. In these patients, hypertensive and other forms of atherosclerotic diseases like coronary and/or peripheral artery involvements are likely to be present before the manifestation of RAS. The uncommon etiology of RAS is large-vessel vasculitis, accountable for less than 1% of cases (12).
In a patient who has a kidney with normal function, the administration of angiotensin-converting enzyme II inhibitor (ACEi) or angiotensin receptor blocker (ARB) drugs will reduce systemic blood pressure and prevent microvascular damage to the normal kidney. However, if the artery stenosis is bilateral, normal natriuresis does not occur. In this situation, high blood pressure is mainly due to an increase in intravascular volume, and it is better to prescribe a diuretic for the patient. This condition also occurs when the patient has only one functioning kidney. In these cases, administration of ACEi or ARB may cause acute kidney injury (13). In our case, although the definite diagnosis was severe unilateral RAS with a single functional kidney, the patient had controlled blood pressure upon admission, and the main symptom was abrupt generalized edema.
There are two other clinical symptoms of this disease, including cardiac destabilization syndromes and ischemic nephropathy (14). This disease can also lead to CKD, pulmonary edema, aortic syndromes, stroke, congestive heart failure (CHF), and acute coronary syndrome (ACS) (15). Other clinical symptoms related to RAS include unexplained azotemia, unilateral small kidney, unexplained hypokalemia, abdominal bruit, severe retinopathy, and peripheral vascular disease (16).
There are several paraclinical methods to diagnose RAS. In severe bilateral RAS or unilateral RAS in functional single kidney, serum Cr and blood urine nitrogen (BUN), which are markers of renal failure, may increase, and GFR will reduce (17). In our patient, serum Cr and BUN values were 9.8 mg/dL and 116 mg/dL at the time of admission, which decreased to 3.1 mg/dL and 43.9 mg/dL, respectively, by the time of discharge as a result of revascularization. Several imaging techniques are also used, including CT angiography, MR angiography, ACE inhibition scintigraphy, captopril renography, color-coded duplex ultrasound (DUS), and angiography, with the latter still considered the gold standard for diagnosis of RAS. However, angiography is an invasive procedure and may cause nephropathy and embolic events (1, 17). Patients with unilateral or bilateral RAS and hypertension should receive antihypertensive therapy. In these patients, ACEis and ARBs can be used (18, 19). Our patient was diagnosed with a known case of hypertension, so he was receiving multiple anti-hypertensive drugs (mentioned earlier) and was normotensive at the time of admission. In some patients, revascularization is suggested via percutaneous transluminal renal angioplasty (PTRA) or surgery based on the etiology and severity of the disease and the possibility of regaining normal kidney function (20).
In this case, we performed an angiography to detect stenosis in the left renal artery on the fifth day of admission. It showed 99% stenosis at the origin of the left renal artery. Immediately after, the patient was considered for revascularization through percutaneous transluminal angioplasty and stenting. The procedure resulted in a rapid decline in serum Cr and urea levels, substantial improvement in urinary output, and resolution of the severe edema. The patient is now receiving ambulatory care in the nephrology clinic. During 4 years after discharge, he experienced no episode of hypertension crisis, and his blood pressure was well under control via oral medications. The kidney function was steady, and the mild CKD of the patient (although with one functional kidney) has not progressed.