1. Background
Urinary tract infection (UTI) is the most prevalent healthcare-associated infection worldwide, consisting of about 40% of all healthcare-associated infections (1-3). In Iran, it was reported as the cause of 32% of all healthcare-associated infections (4).
It could lead to major problems in hospitalized patients, including the increase in the length and the cost of hospitalization and the inappropriate administration of antibacterial (5, 6).
Due to extensive prescriptions of broad-spectrum antibiotics in the hospitals, bacteria resident in the hospital have altered from sensitive bacteria to more resistant ones (7, 8).
Treatment of healthcare-associated UTI (HA-UTI) is usually started empirically and before the preparation of the laboratory results of urine culture. The prevalent causes of the infection and their antibiotic susceptibility pattern show a wide geographic variation (9). Therefore, physicians should know the etiologic agents and their antibiotic sensitivity patterns of this infection in each region (3, 10).
Despite the high prevalence and paramount importance of HA-UTI, few comprehensive investigations have been done on this topic in recent years.
In many studies that have been previously conducted in this field, urine cultures have been studied regardless of the clinical and laboratory findings of UTI. Therefore, contaminant isolates are included in final reports (7, 9, 11-14).
Moreover, in several studies, samples were taken from a small subgroup of patients, such as patients with uncomplicated HA-UTI (13), or patients with bacteremic HA-UTIs (15), or patients admitted to a specific hospital ward (1, 11, 14).
The limitations noted above have rendered it impossible to generalize the results of these studies to all patients with HA-UTIs. However, there are limited studies in which the clinical identification of patients with HA-UTIs has been made properly, and the prevalence and sensitivity of the isolates have been studied on different subgroups of patients (6, 16).
2. Objectives
Considering the lack of comprehensive research on HA-UTIs and the lack of such studies in the region, this study aimed to determine the etiological agents of HA-UTIs and their resistance to the commonly used antibiotics in Isfahan, Iran.
3. Methods
3.1. Study Design
The purpose of this survey was to collect the results of the types and antibiotic susceptibility of pathogens in the individuals who were diagnosed with HA-UTI. The survey was designed to examine the principal microorganisms and their antibiotic susceptibility profile in patients with various infections admitted to three main referral hospitals in Isfahan, Iran. Besides investigating the antibacterial resistance of clinical isolates, the study intended to exclude the contaminant isolates and to confirm the nosocomial origin of the infection with the assistance of experienced infectious control nurses and physicians in the hospitals. The medical centers that were included in the survey were Al-Zahra, Dr. Shariati, and Dr. Gharazi hospitals.
Urine specimens were taken from urinary catheters of the patients with a suspected diagnosis of HA-UTI. According to the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) definitions, HAIs are infections occurring in patients during the process of care in a hospital that was not present or incubating at the time of admission. Fever or lower urinary tract symptoms such as foul-smelling urine or pain in hypogastric areas were regarded as suggestive symptoms of HA-UTI. The UTI was described as the existence of pyuria in the urine sample (≥ 10 WBC/hpf), coupled with the growth of at least 10 5 colonies of a single urinary pathogen in one urine sample or a single non-urinary pathogen in 2 urine specimens with a similar susceptibility pattern. Patients with community-acquired UTI were excluded.
3.2. Organism Identification and Antimicrobial Susceptibility Testing
Pathogens were isolated and identified by routine and conventional techniques. The disk diffusion method was used to detect the sensitivity to antimicrobials according to the guidelines of the Clinical Laboratory Standard Institute (17, 18). The sensitivity of isolates was assessed to the following class of antibiotics: aminoglycosides (gentamicin 10 µg or amikacin 30 µg), cephalosporins (cefotaxime 30 µg or ceftriaxone 30 µg or cefepime 30 µg and ceftazidime 30 µg and cefepime 30 µg), fluoroquinolones (ciprofloxacin 5 µg or levofloxacin 5 µg or ofloxacin 5 µg), folate inhibitors (trimethoprim-sulfamethoxazole 1.25/23.75 µg), carbapenems (imipenem 10 µg or meropenem 10 µg), tetracyclines (tetracycline 30 µg), glycopeptides (vancomycin 30 µg), nitrofurantoins (nitrofurantoin 300 µg), penicillins (penicillin 10 units, Ampicillin 10 µg), and oxazolidinones (linezolid 30 µg). Dehydrated antibiotic discs were commercially prepared from MAST, Merseyside, UK. The minimum inhibitory concentration (MICs) of vancomycin was determined by the E-test strips (Liofilchem, Roseto Degli Abruzzi, Italy) according to the manufacturer's instructions. The kits and techniques were similar in all three listed laboratories.
3.3. Statistical Analysis
Data on sex and age (< or = 20 years, > 20 years) groups, as well as etiology and antibiotic sensitivity, were extracted from WHONET v 5.6 software per laboratory and examined with SPSS version 18.0. To compare the etiology and antibacterial sensitivity in different groups, chi-square or Fisher exact tests were used. A P-value of less than 0.05 was regarded as significant.
3.4. Ethical Consideration
The protocols of this research were evaluated and confirmed by the Institutional Review Board of Isfahan University of Medical Sciences, Iran (approval number: IR.MUI.MED.REC.1399.329).
4. Results
In total, 5,844 urine samples were received for culture, of which 3,937 (67.4%) samples did not show any growth. Besides, the growth of yeast was reported in 284 (4.9%) specimens. From 1623 (27.8%) cases that showed growth of bacteria, 511 (8.7%) samples were considered as contamination, and 1180 (20.2%) bacteria were diagnosed as community-acquired isolates. Finally, 283 HA-UTI cases were detected, of which 130 (45.9%) were male and 32 (11.3%) were under 20 years old.
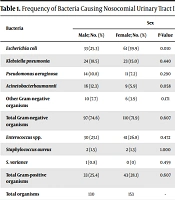
The investigation revealed that Gram-negative bacteria comprised 73.1% (207) of the isolates. Among them, Escherichia coli (45.4%) was the most prevalent bacteria followed by Klebsiella pneumonia (22.7%), Acinetobacter baumannii (12.1%), Pseudomonas aeruginosa (12.1%), and other Gram-negative rods including Acinetobacter spp., Citrobacter freundii, Klebsiella aerogenes, Klebsiella oxytoca, Proteus mirabilis, and Enterobacter spp. (7.7%). The prevalence of E. coli was greater in females compared to males (Table 1).
| Bacteria | Sex | Age Group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P-Value | OR (95% CI) | < 20 | > 20 | P-Value | OR (95% CI) | ||
| Escherichia coli | 33 (25.3) | 61 (39.9) | 0.010 | 1.949 (1.170 - 3.248) | 6 (18.8) | 88 (35.1) | 0.065 | 2.339 (0.928 - 5.899) | 94 (33.2) |
| Klebsiella pneumonia | 24 (18.5) | 23 (15.0) | 0.440 | 0.781 (0.418 - 1.462) | 13 (40.6) | 34 (13.5) | < 0.001 | 0.229 (0.104 - 0.506) | 47 (16.6) |
| Pseudomonas aeruginosa | 14 (10.8) | 11 (7.2) | 0.290 | 0.642 (0.281 - 1.467) | 0 (0) | 25 (10) | 0.091 | 1.142 (1.090 - 1.195) | 25 (8.8) |
| Acinetobacterbaumannii | 16 (12.3) | 9 (5.9) | 0.058 | 0.445 (0.190 - /1.045) | 3 (9.4) | 22 (8.8) | 1.000 | 0.929 (0.262 - 3.296) | 25 (8.8) |
| Other Gram-negative organisms | 10 (7.7) | 6 (3.9) | 0.171 | 0.490 (0.173 - 1.386) | 1 (3.1) | 15 (6) | 1.000 | 1.970 (0.251 - 15.437) | 16 (5.7) |
| Total Gram-negative organisms | 97 (74.6) | 110 (71.9) | 0.607 | 0.870 (0.513 - 1.478) | 23 (71.9) | 184 (73.3) | 0.863 | 1.075 (0.473 - 2.439) | 207 (73.1) |
| Enterococcus spp. | 30 (23.1) | 41 (26.8) | 0.472 | 1.220 (0.709 - 2.099) | 9 (28.1) | 62 (24.7) | 0.674 | 0.838 (0.368 - 1.908) | 71 (25.9) |
| Staphylococcus aureus | 2 (1.5) | 2 (1.3) | 1.000 | 1.113 (0.154 - 8.030) | 0 (0) | 4 (1.6) | 1.000 | 1.130 (1.083 - 1.178) | 4 (1.4) |
| S. variance | 1 (0.8) | 0 (0) | 0.459 | 2.186 (1.925 - 2.482) | 0 (0) | 1 (0.4) | 1.000 | 1.128 (1.082 - 1.176) | 1 (0.4) |
| Total Gram-positive organisms | 33 (25.4) | 43 (28.1) | 0.607 | 1.149 (0.677 - 1.951) | 9 (28.1) | 67 (26.7) | 0.863 | 0.931 (0.410 - 2.112) | 76 (26.9) |
| Total organisms | 130 | 153 | - | - | 32 | 251 | - | - | 283 |
Frequency of Bacteria Causing Nosocomial Urinary Tract Infection in Patients Hospitalized in 3 Hospitals According to Sex and Age Groups in Isfahan, Iran a
On the other hand, among 76 Gram-positive organisms, Enterococcus spp. comprised 93.4% of the isolates, followed by Staphylococcus aureus and Streptococcus viridans (1.8%) (Table 1).
Table 2 demonstrates the sensitivity of the main cause of HA-UTI. As it presents, among Gram-negatives, in contrast to E. coli that had a high sensitivity to imipenem/meropenem (98.2%), amikacin/gentamicin (90.7%), and nitrofurantoin (88.2%), the sensitivity of non-E.coli strains to most studied antibiotics were low. Moreover, it elucidates that Enterococcus spp., the most prevalent Gram-positive isolate, was highly sensitive to linezolid (100%) and nitrofurantoin (72.0%) and highly resistant to other studied antibiotics, including vancomycin (38.5%), ampicillin (34.3%) and penicillin (30.6%).
| Antibiotic Sensitivity | Etiology (Total Isolates); n/N (%) a | ||||
|---|---|---|---|---|---|
| Escherichia coli (94) | Klebsiella pneumonia (47) | Acinetobacter baumannii (25) | Pseudomonas aeruginosa (25) | Enterococcus spp. (71) | |
| Cefotaxime or ceftriaxone or ceftazidim | 51/148 (34.4) | 16/63 (25.4) | 1/31 (3.2) | 9/23 (39.1) | - |
| Cefepime | 41/85 (48) | 8/40 (20) | 0/22 (0) | 9/25 (36) | - |
| Imipenem or meropenem | 110/12 (98.2) | 26/49 (53.1) | 2/26 (7.7) | 12/32 (37.5) | - |
| Amikacin or gentamicin | 110/122 (90.7) | 24/51 (47) | 5/25 (20) | 15/23 (65.2) | 3/5 (60) |
| Ciprofloxacin | 36/89 (40.4) | 7/32 (21.9) | 1/13 (7.7) | 8/23 (34.8) | 7/64 (10.9) |
| Trimethoprime- sulfamethoxazol | 21/76 (27.6) | 13/40 (32.5) | 3/9 (33.3) | 0/8 (0) | - |
| Nitrofurantoin | 30/34 (88.2) | 4/10 (40) | 0/1 (0) | 0/4 (0) | 36/50 (72) |
| Ampicillin | 3/27 (11.1) | 0/6 (0) | - | - | 24/70 (34.3) |
| Vancomycin | - | - | - | - | 25/65 (38.5) |
| Linezolid | - | - | - | - | 37/37 (100) |
| Penicillin G | - | - | - | - | 19/62 (30.6) |
| Tetracycline | - | - | - | - | 13/63 (20.6) |
Antimicrobial Sensitivity of Major Isolates Causing Healthcare-Associated Urinary Tract Infection in Patients Hospitalized in Three Hospitals According to Sex and Age Groups in Isfahan, Iran
The study unveiled the most effective antibiotics against Gram-negative bacteria to be nitrofurantoin (69.8%), carbapenems (69.7%), and aminoglycosides (68.6%) followed by cefepime (34.2%), fluoroquinolones (32.3%), trimethoprim-sulfamethoxazole (29.1%), and third generation cephalosporins (28.9%). The sensitivity of Gram-negative isolates to carbapenems, aminoglycosides, fluoroquinolones, and third-generation cephalosporins was significantly higher in females than males. Furthermore, susceptibility to aminoglycosides and third-generation cephalosporins was greater among the cases older than 20 than those under 20 years of age (Table 3).
| Antibiotic | Sex | Age Group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P-Value | OR (95% CI) | < 20 | > 20 | P-Value | OR (95% CI) | ||
| Cefotaxime or ceftriaxone or ceftazidim | 25/130 (19.2) | 57/153 (37.3) | 0.001 | 2.494 (1.445 - 4.303) | 4/32 (12.5) | 78/251 (31.1) | 0.037 | 3.156 (1.070 - 9.305) | 82/283 (28.9) |
| Cefepime | 25/92 (27.2) | 41/101 (40.6) | 0.050 | 1.831 (0.998 - 3.361) | 3/21 (14.3) | 63/172 (36.6) | 0.051 | 3.468 (0.983 - 12.238) | 66/193 (34.2) |
| Imipenem or meropenem | 66/110 (60) | 102/131 (77.9) | 0.003 | 2.345 (1.337 - 4.112) | 17/25(68) | 151/216 (69.9) | 0.844 | 1.093 (0.449 - 2.660) | 168/241 (69.7) |
| Amikacin or gentamicin | 69/113 (61.1) | 97/129 (75.2) | 0.018 | 1.933 (1.115 - 3.351) | 11/25 (44) | 155/217 (71.4) | 0.005 | 3.182 (1.370 - 7.391) | 166/242 (68.6) |
| Ciprofloxacin or ofloxacin or levofloxacin | 21/88 (23.9) | 40/101 (39.6) | 0.021 | 2.092 (1.112 - 3.936) | 2/11 (18.2) | 59/178 (33.1) | 0.508 | 2.231 (0.467 - 10.656) | 61/189 (32.3) |
| Trimethoprim- sulfamethoxazole | 18/67 (26.9) | 25/81 (30.9) | 0.594 | 1.215 (0.592 - 2.489) | 4/20 (20) | 39/128 (30.5) | 0.433 | 1.753 (0.550 - 5.583) | 43/148 (29.1) |
| Nitrofurantoin | 17/24 (70.8) | 20/29 (69) | 0.883 | 0.915 (0.281 - 2.979) | 3/3 (100) | 34/50 (68) | 0.545 | 0.919 (0.835 - 1.011) | 37/53 (69.8) |
The study also confirmed that the sensitivity of Gram-positive strains to linezolid (100%) and nitrofurantoin (74.5%) was high and to ampicillin (33.8%), vancomycin (27.7%) and ciprofloxacin (13.2%) was low. The susceptibility of these isolates to the studied antibacterial agents was not significantly different in age and sex categories (Table 4).
| Antibiotic | Sex | Age Group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P-Value | OR (95% CI) | < 20 | > 20 | P-Value | OR (95% CI) | ||
| Ampicillin | 10/31 (32.3) | 14/40 (35) | 0.809 | 1.131 (0.418-3.057) | 2/9 (22.2) | 22/62 (35.5) | 0.708 | 1.925 (0.368 - 10.077) | 24/71 (33.8) |
| Nitrofurantoin | 14/19 (73.7) | 27/36 (75) | 0.915 | 1.071 (0.301-3.814) | 4/6 (66.7) | 37/49 (75.5) | 0.638 | 1.542 (0.250 - 9.496) | 41/55 (74.5) |
| Ciprofloxacin | 4/28 (14.3) | 5/40 (12.5) | 1.000 | 0.857 (0.208-3.524) | 0/2 (0) | 9/66 (13.6) | 1.000 | 1.035 (0.987 - 1.086) | 9/68 (13.2) |
| Linezolid | 16/16 (100) | 22/22 (100) | - | - | 6/6 (100) | 32/32 (100) | - | - | 38/38 (100) |
| Vancomycin | 12/44 (27.3) | 16/57 (28.1) | 0.929 | 1.041 (0.432 - 2.508) | 2/16 (12.5) | 26/85 (30.6) | 0.223 | 3.085 (0.654 - 14.559) | 28/101 (27.7) |
5. Discussion
This study demonstrated that E. coli, Enterococcus spp., K. pneumonia, P. aeruginosa, and A. baumannii were the most common caucuses of HA-UTIs in this geographical region. Additionally, it was demonstrated that non-E. coli strains were more prevalent in males compared to females and revealed that the prevalence of K. pneumonia was higher in those under 20 years of age.
Moreover, this research showed that the sensitivity of the isolated Gram-negative bacteria, especially A. baumannii, P. aeruginosa, and K. pneumonia strains, to the studied antibiotics was so low that the overall sensitivity of Gram-negative bacteria to potent antibiotics such as nitrofurantoin, carbapenems, and aminoglycosides were only about 70% and had a much lower sensitivity to trimethoprim-sulfamethoxazole, fourth and third generation cephalosporins, and fluoroquinolones.
Besides, the Gram-negative bacteria sensitivity to carbapenems, aminoglycosides, fluoroquinolones, and third-generation cephalosporins was higher in females than males, and sensitivity to aminoglycosides and third-generation cephalosporins was higher in those over 20 than less than 20 years of age. Also, this study indicated high resistance of the isolated Gram-positive bacteria to ampicillin, ciprofloxacin, and vancomycin and high sensitivity to linezolid and nitrofurantoin.
Overall, the study showed that a carbapenem and an aminoglycoside can be used for the empiric treatment of severe HA-UTI in the study region. In very severe cases, linezolid can also be added to treat possible Gram-positive strains.
In this study, the most common cause of HA-UTIs was E.coli, followed by Enterococcus spp., K. pneumonia, P. aeruginosa, and A. baumannii. According to the majority of studies conducted in this field, E. coli is the most common causative agent of the infection (1, 6, 9, 11, 13, 15, 16) except for a study conducted in India (14) in which the most prevalent agent was proven to be K. pneumonia and then E. coli. Other common bacterial agents in other areas were similar to our findings, and the differences in various studies were merely in their positions and rankings in the list of causative agents (1, 6, 9, 11, 13-16).
This research demonstrated that non-E.coli strains, most of which are antibiotic-resistant organisms, were more prevalent in males than females. The majority of the studies on HA-UTIs did not adopt a gender-specific approach. In other words, the number of microorganisms and the antibiotic sensitivity of microorganisms were not separately investigated in males and females. However, in a similar study in Tehran (7), Iran, the percentage of non-E. coli strains in both genders were equal. This finding indicates that in our region, in the initial treatment of such infections in males, the possibility of infection with non-E. coli resistant strains, and consequently, a more effective antibiotic treatment or combination of antibiotics should be considered.
Our study revealed that the prevalence of K. pneumonia was higher in patients under 20 than in those over 20 years old. Unfortunately, none of the studies on the same subject made any attempt to differentiate microorganisms by age group. Due to the high prevalence of antibiotic resistance in K. pneumoniae strains compared to E. coli, empiric antibiotic treatment in patients under the age of 20 should include more potent antibiotics.
This research revealed that the sensitivity of Gram-negative strains in HA-UTI to fourth and thrid generation cephalosporins, including cefotaxime, ceftriaxone, ceftazidime, and cefepime, is very low and less than 35%. Therefore, this group of antibiotics is not suitable and not recommended to treat HA-UTI in this geographical region. Our study is similar to a study conducted in southern Iran (6) that reported similar antibiotic sensitivity results to 3rd and 4th generation cephalosporins. Other studies conducted in Tehran (1, 7) in 2009 - 2010 and 2010 - 2014 had shown that the sensitivity of pathogens to the above cephalosporins is about 60%. Regarding other countries, the sensitivity to this class of drugs in Turkey was approximately 70% (16), and in India, it was 90% in one study (14) and 70% in another (13) The heterogeneity of the findings could be due to the geographical diversity of the microbial types and their resistance.
Evidence shows that the most effective known antibiotics for the empiric treatment of HA-UTIs are carbapenems and aminoglycosides. Our findings revealed that approximately 70% of strains were sensitive to these classes of antibiotics. This finding has also been reported in most previous articles. Considering the synergistic effect of carbapenems and aminoglycosides against Gram-negative bacteria, it seems that simultaneous treatment with these two classes of antibiotics can increase the chances of responding to the treatment. This combinational therapy can be advised in severe cases, especially in males and patients under the age of 20, when strains are more likely to be multi-drug resistant.
On the other hand, the study revealed that the sensitivity of isolated Gram-negative and Gram-positive strains to nitrofurantoin was about 70%, and this drug can be used in mild cases of infection when the patients can be treated orally. In other studies, sensitivity to this drug has been reported to be different, which may be attributed to different antibiotic usage in different places (1, 6, 7, 12-14).
In this study, the resistance of Gram-negative bacteria to fluoroquinolones, including ciprofloxacin, ofloxacin, and levofloxacin, was observed to be very high, and more than 67% of bacteria were resistant to these antibiotics. Thus, the use of fluoroquinolones for the empiric treatment of HA-UTI in our region is not recommended. The majority of other similar studies also reported a high sensitivity to these antibiotics (1, 6, 7, 12, 13, 16). It is noteworthy that in a study conducted in India (14), high resistance to this class of drugs was reported. One of the reasons for the high sensitivity to these drugs in some regions and high resistance in others can be the consumption rate of the mentioned antibiotics across communities, resulting in the induction of microbial resistance among microorganisms in that region.
The study reported a 30% sensitivity of Gram-negative organisms to trimethoprim-sulfamethoxazole, which shows that this drug is not suitable for the initial treatment of HA-UTI in the region under study. In other articles that had reported the antimicrobial susceptibility of this drug, a sensitivity of about 50% was reported (1, 6, 7, 9, 11-13, 16). Therefore, it seems that this antibiotic is not suitable for the initial treatment of HA-UTI in most geographical regions.
This study indicated that the sensitivity of Gram-negative isolates to third generation cephalosporins, carbapenems, aminoglycosides, and fluoroquinolones was higher in females than in males. The greater level of resistance in the male population is probably due to the higher prevalence of non-E. coli strains such as K. pneumonia, P. aeruginosa, and A. baumannii is the most multidrug-resistant strain in male individuals.
In Gram-positive bacteria, resistance to vancomycin, ampicillin, and ciprofloxacin was so high that less than 35% of the isolates were sensitive to these antibiotics. These antibacterial agents are not suitable against Gram-positive causes of HA-UTIs. In most previous articles, there was no intention to assess the antibiotic susceptibility of isolates by Gram-staining groups separately. However, in a study in southern Iran (6), the resistance of Gram-positive isolates was studied, and high resistance to ampicillin and very low resistance to vancomycin was reported. The reliability of the report, however, is low due to the limited number of studied Gram-positive strains. In another similar study conducted in Tehran (7), high resistance of Gram-positives to ampicillin was reported.
Moreover, according to our research, all isolated Gram-positive were sensitive to linezolid, and this drug can be used for the coverage of Gram-positive bacteria in the initial treatment of HA-UTI. However, application of this antibiotic for empiric therapy should be limited to severe infections. It is also necessary to take stewardship measures to prevent nosocomial bacteria from becoming resistant to this drug and discontinuing or changing the type of antibiotic used should be considered after determining the results of the urine culture.
The limitations of this research should also be noted. First, this research was conducted only in three referral hospitals in Isfahan province, which are not representative of all the hospitals in the province or the country. Nevertheless, considering that in recent years, almost no similar research has been done in the central parts of Iran, this study can be considered a primary guide for the treatment of patients with HA-UTI in this region. Similar studies in other parts of the country and other non-referral hospitals in the province should be conducted to determine the appropriate empiric antibiotic approach.
The second limitation of the research pertained to the practical aspects of conducting it. As this study was performed during the routine work of clinical laboratories and the choice of the antibiotic kit of the laboratory was based on the type of kit that was available in the laboratory at the time of bacterial extraction, it was not possible to investigate the microbial sensitivity to all antibiotics for all isolated microorganisms.
Despite all mentioned limitations, it should be noted that this research can be considered of particular importance for the following reasons. First, in this study, accurate definitions of nosocomial infection, contamination of the culture medium, and urinary tract infection are presented, demonstrating the strength of the study in identifying real strains of HA-UTI. Second, drug resistance investigation by age, gender, and Gram staining, besides the bacterial strain types, which were considered in this research, are of particular novelty.
5.1. Conclusions
This study showed that the resistance of Gram-negative bacteria causing HA-UTI to cephalosporins, quinolones, and trimethoprim-sulfamethoxazole was very high, and these antibiotics are not suitable for the initial treatment. On the other hand, the study indicated that Gram-positive bacterial causes of HA-UTI in this region were highly resistant to vancomycin, ampicillin, and ciprofloxacin, and these antibiotics are not suitable for the empiric treatment of this infection. In addition, our study indicated that the total sensitivity of isolated bacteria, both Gram-negative and Gram-positive, to nitrofurantoin, is relatively high, and this drug can be used in mild cases of the disease that can be treated orally.