1. Background
Measurement of the outcomes of patients admitted to intensive care units (ICUs) is difficult, but very important. It contains several challenges for research outcomes. Much progress and developments are made in this field as technological and therapeutic aspects. The main aims of health care units are to decrease mortality and morbidity, and also keep up or make the functional status and quality of life better for the patients admitted to ICU (1).
Electrolyte imbalance is one of the key factors that play an important role in this context. Among these factors, abnormalities in the levels of magnesium (Mg) in serum can be noted. Previous studies in this field reported that the incidence of hypomagnesemia in hospitalized patients was about 10% to 15% and about 60% in the patients admitted to ICUs (2).
Magnesium has essential roles in most cellular reactions. It acts as a cofactor in up to 300 metabolic reactions such as glycolysis, fat and protein metabolism, ATP synthesis, and the second messenger system (3-6). Moreover, it plays a role in muscle function including muscle contraction, protein synthesis, production of energy and liquid balance. Magnesium is a physiologic regulator in cell membrane integrity and contributes to the function of neuromuscular, cardiovascular, immunologic, and hormonal systems (7, 8). Oxidative stress and impaired intracellular calcium homeostasis due to hypomagnesemia cause structural damage of muscle cells (9, 10).
Most of the energy used in physiologic function in humans is produced by mitochondria and displacement of electrons in the respiratory cycle. Magnesium is necessary for essential mitochondrial function including synthesis of the lower units of electron transfer complex, ATP synthesis, and detoxification of oxygen. Insufficiency of Mg decreases mitochondrial function and increases the production of free radicals by distorted skeletal cells, function of proteins, DNA, and other vital molecules (11). Hypomagnesemia is a usual finding in patients admitted to ICU that can be caused by Mg loss in kidney or intestine.
Hypomagnesemia has different causes including insufficient intake of Mg, malabsorption, gastrointestinal losses (diarrhea, vomiting, and laxative abuse), renal losses (congenital or acquired tubular defects), drugs (diuretics, angiotensin-converting-enzyme (ACE) inhibitors, aminoglycosides, amphotericin, and cyclosporine), endocrine disorders (hyperaldosteronism, hyperparathyroidism, hyperthyroidism, and diabetes), and other causes such as alcoholism, excessive sweating, and extensive burns (12).
Hypomagnesemia has many manifestations and complications including cardiovascular complications (atrial fibrillations, ventricular arrhythmia, and torsade de point), neurologic complications (the Chvostek sign, the Trousseau sign, tetany, and muscle cramps), and other electrolyte abnormalities often observed with hypomagnesaemia (hypocalcemia and hypokalemia); also, hypomagnesemia is one of the important causes of disturbance in ventilator weaning (13). Furthermore, hypomagnesemia can increase the severity of disease, mortality and morbidity. Some studies showed that hypomagnesemia may lead to the increased risk of mortality (14).
In a case-control study conducted on 70 patients, data of age, gender, type of stroke, and serum levels of Mg and potassium (K) were recorded and the results were compared with those of the control group. The results showed that the mean of serum levels of Mg and K were lower in patients than the control group. The serum level of Mg and K were lower in patients with cerebrovascular accident (CVA) than the control group (15). Tong and Rude et al., showed that mortality rate was higher in patients with hypomagnesemia who admitted to ICU (1). In the study by Huijgen et al., the level of Mg in serum was measured in 115 patients in ICU. The mean of serum level of Mg in the patients admitted to ICU was 1.9 mg/dL and the frequency of hypomagnesaemia was reported 51.3% (16).
2. Objectives
The current study aimed at determining the incidence of abnormalities in the serum levels of Mg in patients admitted to ICU to evaluate the association of these variables with factors associated with the outcome of the patients such as cardiac, renal, gastrointestinal (GI) tract, pulmonary, endocrine, and oncologic dysfunctions and complications.
3. Methods
3.1. Patients
The current cross sectional study was undertaken in all of the patients admitted and stayed at least 2 days in Shahid Beheshti hospital ICU from February 2011 to March 2012. A total of 101 patients who met the inclusion criteria were enrolled in the study. Exclusion criteria were as follows: hemoglobin level of less than 10 g/dL, receiving blood products, Mg or Ca infusion before sampling, and receiving oral Mg supplementations. Moreover, the patients who were discharged in the day 1 of ICU stay were excluded.
To avoid the selection bias, the patients who fulfilled the criteria for critical illness and were newly admitted to ICU were enrolled. The level of Mg in serum was measured in such patients. In all cases, a complete history was taken, physical examination was performed, and findings related to the disease were evaluated.
All characteristics of the patients including age, gender, medical history, vital signs, route of feeding in ICU, medical history related to etiology (including GI disease, hypertension, and diabetes), drug abuse (especially diuretics, ACE inhibitors, and chemotherapy drugs), and blood pH were recorded.
Oral and parenteral Mg intake, medication use, severe diarrhea, steatorrhea, and smoking were statistically considered as confounding variables within the regression. Also, overestimation, underestimation, and poor recall might have confounded the results.
In the third day of admission, electrolyte profile including serum levels of Ca, sodium (Na), K, Mg, phosphor (P), blood urea nitrogen (BUN), and creatinine (Cr) were measured. After draining 5 mL of blood, clotting, and centrifuging, the separated serum was collected at -20°C, and Mg level was measured by the xylidyl blue method. The normal range of Mg is 1.8 to 2.5 mg/dL. Serum Mg level of < 1.70 mg/dL was considered as hypomagnesemia and ≥ 2.56 mg/dL as hypermagnesemia.
The results of laboratory testing, medical history, physical examinations, and findings related to the disease were recorded in a checklist. All of the patients were followed-up until discharge from the hospital.
The primary endpoint was to investigate the association of serum levels of Mg and mortality. Secondary endpoints included the relationship between Mg changes and clinical factors associated with the outcomes of patients admitted to ICU.
3.2. Ethical Considerations
The study was completely conducted in accordance with the declaration of Helsinki and approved by the research and ethics committee of Hamadan University of Medical Sciences, Hamadan, Iran. The purpose of the study was explained to all participants. A written informed consent was signed by the first-degree relatives of the patients admitted to ICU in the presence of 2 witnesses.
3.3. Statistical Analysis
Data were transferred to excel microsoft software 2007 and reviewed via software package used for statistical analysis (SPSS), version 18. Descriptive analysis of quantitative and qualitative data was presented as mean ± standard deviation (SD). Also, nominal qualitative data were compared using Chi-square test and the mean difference of quantitative variable analyzed by the Mann-Whitney U test. Regression analysis was performed to determine the effect of significant independent risk factors on mortality in ICU. Significance level was determined ≤ 0.05.
4. Results
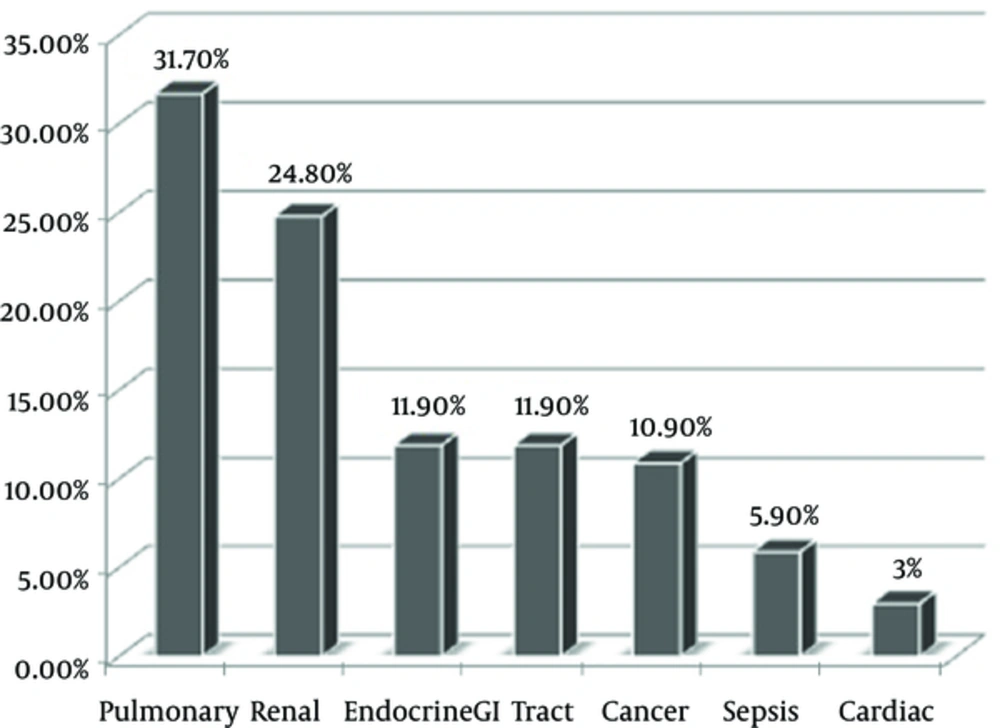
The current study was performed on 101 patients with the mean age of 68.07 ± 17.73 years, ranged from 15 to 90 year, in which 51.5% were male (n = 52). Most of the patients had pulmonary 31.7% (17) and renal 24.8% (18) disorders, respectively (Figure 1).
The mean duration of staying in hospital was 17.55 ± 13.8 days. For 64 patients (63.4%) who underwent mechanical ventilation, the mean duration of ventilation was 12.87 ± 10.41 days. The route of feeding was enteric feeding in 49.5%, oral feeding in 33.7%, fasting in 15.8%, and parenteral in 1% of the patients. In all patients, the serum levels of Ca, Na, K, and, Mg were measured (Table 1).
| Mean ± SD | Decreased, N (%) | Normal, N (%) | Increased Level, N (%) | |
|---|---|---|---|---|
| Sodium (Na) | 139.51 ± 6.48 | 18 (17.8) | 70 (69.3) | 13 (12.9) |
| Potassium (K) | 4.34 ± 0.84 | 9 (8.9) | 85 (84.2) | 7 (6.9) |
| Magnesium (Mg) | 2.16 ± 0.79 | 32 (31.7) | 51 (50.5) | 18 (17.8) |
Also, blood pH was evaluated in the patients, and the amount was low in 9 (8.9%), normal in 47 (46.5%), and high in 45 (44.6%) patients. No significant difference was found between the serum Mg and pH (P value = 0.5).
Hypomagnesemia and hypermagnesemia existed in 31.7% and 17.8% of the patients, respectively. Also, hypomagnesaemia was almost observed in patients with GI (50%) and renal (36%) diseases (Table 2)
| Clinical Problems | Hypomagnesaemia, N (%) | Normal Magnesium, N (%) | Hypermagnesemia, N (%) | Total, N (%) |
|---|---|---|---|---|
| Cardiac | 1 (33) | 2 (67) | 0 (0) | 3 (100) |
| Renal | 9 (36) | 13 (52) | 3 (12) | 3 (100) |
| GI tract | 6 (50) | 4 (33) | 2 (17) | (100) |
| Pulmonary | 9 (28) | 17 (53) | 6 (19) | 32 (100) |
| Endocrine | 3 (25) | 8 (67) | 1 (8) | 12 (100) |
| Cancer | 3 (28) | 4 (36) | 4 (36) | 11 (100) |
| Sepsis | 1 (17) | 2 (50) | 2 (33) | 6 (100) |
No significant difference was observed between the serum level of Mg and the etiology of admission (P value = 0.075). Also, there was no significant difference between serum level of Mg and age (P value = 0.807), duration of staying in hospital (P value = 0.482), and duration of mechanical ventilation (P value = 0.947).
Hypomagnesemia was observed in 30.7% of male and 17.8% of female cases, and hypermagnesemia in 21.1% of male and 14.2% of female cases. The relationship between the serum Mg level and gender was insignificant (P value = 0.528).
No significant difference was observed between the serum level of Mg and serum level of Na (P value = 0.833), Ca (P value = 0.131), and K (P value = 0.205).
Finally, 71 patients (70.3%) died and 30 (29.7%) patients were discharged. Hypomagnesaemia existed in 28.1% of the expired patients and 40% of the discharged ones. There was no significant difference between hypomagnesaemia and the outcomes of the patients (P value = 0.067).
5. Discussion
The current study found no relationship between the changes in the levels of Mg and factors associated with the outcomes of the patients admitted to ICU.
Magnesium, after K, is the second important intracellular cation and plays important roles in transfer, storage, and production of energy used in the body, and affects the regulation and catalysis of 300 vital enzymes (1, 19). Also, Mg is a necessary cofactor in the major enzymatic processes. The physiological and pharmacological roles of this ion in disorders were found in recent years (20). Hypomagnesaemia is the serum Mg level ≤ 1.6 mg/dL and/or depletion of Mg level > 2 SD of the mean of serum Mg level in the general population (21, 22).
The total serum mg level by the laboratory testing in different studies was reported 1.8 to 2.8 mg/dL (23).
Hypomagnesemia is evaluated in patients admitted to ICU in different hospitals. In the conducted studies, hypomagnesemia varied from 9.4% in the severely ill patients due to chronic obstructive pulmonary disease (COPD) to 61% in patients who had undergone major surgeries (24, 25). The risk of mortality in the patients admitted to ICU was 2 to 3 times higher in patients with hypomagnesaemia compared to the ones without hypomagnesaemia (4, 14, 18). Moreover, several studies reported the relationship between hypomagnesaemia and the higher risk of mortality (26, 27).
The current study evaluated this relationship in Iranian patients. In the current study, hypomagnesaemia was found in 31.7% of the subjects that is similar to those of other studies.
In a study conducted in Brazil by Dehinzen et al., the level of Mg in the serum was measured in 226 patients with cancer admitted to ICU. The frequency of hypomagnesemia was reported about 46% (26). Also, hypomagnesemia was reported 61% in the severely ill patients in ICU by Chernow et al., in America (24). Despite the high prevalence of hypomagnesemia in such patients in the current study, there was no relationship between the level of Mg in the serum and mortality (P value = 0.67). Huijgen et al., reported no relationship between serum Mg level and mortality in the severely ill patients, but in a more recent study, Soliman et al., reported a significant relationship between ionized hypomagnesemia and increased mortality (16, 28). As more than 99% of Mg is intracellular and Mg ion is a cofactor in intracellular biochemical process, most of the investigators prefer to evaluate this factor in their studies.
Intracellular ion measurement is better than total serum Mg level for investigations (18, 29). Furthermore, the measurement of serum Mg level alone may not be able to show the hypomagnesemia and there is a need to measure the ionized Mg level in the severely ill patients in ICU. Most of the studies suggested that these complete routes are not measurable and standard; furthermore, the measurement of total serum Mg can be used in patients suspected to hypomagnesaemia, and if in such patients the level of Mg in serum is not determined, other examinations can be used for better (complete) evaluations (30, 31).
In the current study, there was no relationship between the level of Mg in serum, and age and gender, which was similar to the studies conducted by Sabatier et al (17).
In the current study, hypomagnesemia was mostly observed in patients with GI (50%) and renal (36%) diseases, compared with the other patients; therefore, GI tract, skeletal system, and also kidney play significant roles in Mg homeostasis. Intact kidneys and small intestine control the level of Mg in serum through secretion, absorption, and reabsorption to keep the Mg level in a normal limit (22). This physiological key role indicated the need for further studies to identify the role of deficiency of GI absorption and renal loss that was higher in hypomagnesemia (21, 23). The higher prevalence of hypomagnesemia in GI and renal diseases can be caused by the disorders in absorption, reabsorption, and supportive treatment by Mg, which should be done with high accuracy.
In the study by Sabatier et al., there was a significant relationship between the level of Mg in serum and renal disease (P value = 0.025); however, in the current study the difference was insignificant (P value = 0.75) (16, 17). In the current study, there was no significant relationship between the level of Mg in serum and other cations such as Na, K, and Ca, which was similar to the results of previous studies (16, 28, 30).
5.1. Conclusion
The results of the current study showed that one of the major causes of the contrast and different reports on the role of hypomagnesemia in the increase of mortality of patients admitted to ICU was the lack of a standard protocol to measure the level of Mg in serum in such patients. Some other similar studies conducted in this field advised that the measurement of total serum Mg level was preferred to measure the ionized Mg level. Based on the results of the current study, it is advised to perform a thorough study by measuring the ionized Mg level and/or 2 routes together for the better management of patients admitted to ICU, and decrease the mortality and duration of stay in ICU. Also, interventional studies in such cases, especially on the treatments with Mg supplementations, can be useful.