1. Background
As a member of the tissue kallikrein family, prostate-specific antigen (PSA) is a glycoprotein antigen typically produced by the prostate gland's epithelial cells (1). It serves primarily as a diagnostic marker for prostate malignancies (2) and can be found in circulation in various molecular forms: Free and non-complexed PSA (fPSA), with a molecular mass of 28 kDa, and PSA complexed with α-1-antichymotrypsin (cPSA), with a molecular mass of 90 kDa (3). Furthermore, total PSA (tPSA) levels encompass the sum of fPSA and cPSA levels (4). Typically, humans have blood levels of PSA around 0.4 ng/mL, while individuals with prostate cancer exhibit significantly higher levels of this marker (5). Research indicates that prostate cancer affects 30% of men with PSA values between 9.9 and 1.4 ng/mL(6).
The relatively low molecular mass of fPSA, its predicted globular conformation, and its neutral charge at physiological pH all suggest that this molecule is excreted via glomerular filtration. Several studies have reported a half-life of 12 - 18 hours for fPSA, supporting the hypothesis of renal excretion. On the other hand, the excretion pathways of cPSA are not yet fully understood; however, considering its high molecular mass and prolonged elimination rate, evidence suggests elimination mechanisms other than renal clearance for this molecule (7).
In individuals with chronic kidney disease (CKD), the kidneys sustain damage that persists for at least three months, impairing their ability to carry out essential functions. For patients with CKD, interpreting PSA results can be challenging due to the potential impact of renal dysfunction on PSA metabolism and clearance. While some studies have reported higher serum fPSA levels and fPSA rates in patients with severe renal failure undergoing hemodialysis (8, 9), others have not confirmed this association (10, 11). Therefore, it remains uncertain whether the current normal range for PSA should be revised for CKD patients. Such an adjustment could potentially reduce the risk of complications associated with prostate biopsy in this population.
These conflicting findings underscore the need for further investigation into the potential relationship between PSA levels, glomerular filtration rate (GFR), and Body Mass Index (BMI) in CKD patients. If it is confirmed that PSA levels differ in individuals with decreased GFR, aside from age, GFR levels could potentially serve as a threshold to adjust PSA cutoffs for prostate biopsies during prostate cancer screening. This would be particularly relevant given that CKD is a common comorbidity in men with prostate cancer, which may impact the accuracy and interpretation of PSA tests (12).
2. Objectives
In CKD patients, prostate biopsies may be accompanied by several complications, such as sepsis and bleeding, due to impaired kidney function. To mitigate these unwanted consequences, it may be beneficial to reduce the interval between prostate-specific antigen (PSA) tests before biopsy in CKD patients. Therefore, understanding how PSA behaves in CKD patients is crucial for improving diagnostic strategies and clinical management in this population.
3. Methods
This descriptive-analytical study included male patients with CKD who presented to the Urology and Nephrology Clinic of Shohada-e Tajrish Hospital, Tehran, Iran. Inclusion criteria consisted of male patients with a definitive diagnosis of CKD by a nephrologist based on criteria such as proteinuria and GFR reduction, as well as age over 40 years. Exclusion criteria included patients with prostatitis or a history of prostate biopsy, surgery, or cancer. The researcher referred to the urology department of Shohada-e Tajrish Hospital and selected male patients with CKD after the university's ethical council approved the study. Subsequently, the patients were informed about the study's steps and goals. Additionally, all patients were simultaneously assessed for free and total PSA, serum creatinine, and serum total protein levels.
The GFR of all patients was calculated using Cockcroft's formula, considering the patients' age, weight, and urinary creatinine levels. Based on the GFR, patients were divided into three groups: More than 90, 60 - 90, and less than 60. It should be noted that stages 3, 4, and 5 CKD were grouped together. Given the differences in the normal ranges of PSA across various age groups, patients in each age group were divided into two groups: Normal and abnormal, based on their PSA levels. The reason for using this formula was its cost-effectiveness and simplicity compared to other formulas. Furthermore, demographic data of the patients, including age, height, weight, occupation, educational level, and underlying diseases such as diabetes and hypertension, were collected. Additionally, the patients' BMI was calculated using their height and weight. Finally, the relationships between total and free PSA levels with GFR and BMI were analyzed using SPSS software and regression tests, with a significance level set at 0.05.
The present study was approved by the Ethics Committee. All participants were assured of the confidentiality of their data and were allowed to withdraw from the study at any stage. Moreover, the research subjects were briefed about the study goals and procedures before providing written informed consent.
4. Results
The study included 152 male patients with CKD, with a mean age of 58.5 ± 17.6 years. Table 1 displays the distribution of free and total PSA based on GFR. According to our findings, the mean levels of free and total PSA increased as GFR decreased.
| GFR (mL/min/1.73 m2) | Number | Free PSA (95% CI) | Total PSA (95% CI) |
|---|---|---|---|
| ≥ 90 | 64 | 0.23 (0.22 - 0.25) | 0.80 (0.73 - 0.80) |
| 60 - 90 | 72 | 0.28 (0.27 - 0.30) | 0.91 (0.90 - 1.00) |
| 15 - 60 | 16 | 0.45 (0.40 - 0.50) | 1.44 (1.30 - 1.73) |
The mean fPSA level was significantly higher in patients aged 70 - 79 years with a GFR higher than 90 mL/min/1.73 m2 compared to those younger than 70 years with the same GFR range. Additionally, the mean tPSA levels were significantly higher in patients aged 50 - 59 years compared to those aged 40 - 49 years (Table 2). Thus, fPSA and tPSA levels increased with age in patients with a GFR of 60 - 90 mL/min/1.73 m2 and those with a GFR of 15 - 60 mL/min/1.73 m2. However, this increase was only significant for the 60 - 69 years age group (P < 0.004).
| Variable | Free PSA (95% CI) | Total PSA (95% CI) |
|---|---|---|
| Age (y) and GFR | ||
| 40 - 49 | ||
| ≥ 90 | 0.21 (0.19 - 0.23) | 0.70 (0.66 - 0.72) |
| 60 - 90 | 0.22 (0.20 - 0.25) | 0.75 (0.70 - 0.80) |
| 15 - 60 | 0.22 (0.17 - 0.62) | 0.60 (0.33 - 2.65) |
| 50 - 59 | ||
| ≥ 90 | 0.25 (0.23 - 0.27) | 0.89 (0.76 - 1.00) |
| 60 - 90 | 0.26 (0.23 - 0.28) | 0.90 (0.80 - 1.00) |
| 15 - 60 | 0.37 (0.18 - 0.48) | 0.80 (0.66 - 1.38) |
| 60 - 69 | ||
| ≥ 90 | 0.27 (0.24 - 0.29) | 1.03 (0.95 - 1.20) |
| 60 - 90 | 0.37 (0.32 - 0.42) | 1.30 (1.10 - 1.50) |
| 15 - 60 | 0.40 (0.32 - 0.47) | 1.12 (0.90 - 1.58) |
| 70 - 79 | ||
| ≥ 90 | 0.37 (0.32 - 0.47) | 1.50 (1.10 - 1.93) |
| 60 - 90 | 0.45 (0.39 - 0.49) | 1.51 (1.40 - 1.72) |
| 15 - 60 | 0.55 (0.44 - 0.74) | 1.97 (1.30 - 2.61) |
| More than 80 | ||
| ≥ 90 | 0.33 (0.18 - 0.65) | 1.60 (0.90 - 2.49) |
| 60 - 90 | 0.56 (0.50 - 0.68) | 2.10 (1.70 - 2.36) |
| 15 - 60 | 0.62 (0.48 - 0.74) | 2.06 (1.53 - 2.60) |
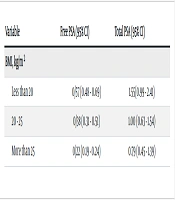
Conversely, free and total PSA levels decreased in CKD patients with increased BMI. The mean total PSA was 1.55 ng/mL in patients with a BMI lower than 20 kg/m2, while it decreased to 0.79 ng/ml in patients with a BMI higher than 25 kg/m2 (Table 3). Additionally, free and total PSA levels increased in patients with decreased serum creatinine clearance (Table 4).
| Variable | Free PSA (95% CI) | Total PSA (95% CI) |
|---|---|---|
| BMI, kg/m2 | ||
| Less than 20 | 0/57 (0.48 - 0.69) | 1.55(0.99 - 2.41) |
| 20 - 25 | 0/38 (0.31 - 0.51) | 1.00 (0.63 - 1.54) |
| More than 25 | 0/22 (0.19 - 0.24) | 0.79 (0.45 - 1.39) |
| Variable | Free PSA (95% CI) | Total PSA (95% CI) |
|---|---|---|
| Creatinine clearance, μmoL/L | ||
| Less than 70 | 0.44 (0.38 - 0.57) | 1.27 (0.71 - 2.25) |
| 70 - 106 | 0.71 (0.63 - 0.82) | 1.91 (0.99 - 3.70) |
| More than 106 | 0.95 (0.88 - 0.99) | 2.15 (0.98 - 4.72) |
5. Discussion
The present study examined the relationship between free and total PSA levels, GFR, and BMI in 152 CKD patients with a mean age of 58.5 ± 17.6 years, who were similar in terms of demographic characteristics. Based on the distribution of free and total PSA levels according to GFR, the mean levels of free and total PSA increased as GFR decreased.
In a study whose results were consistent with ours, Hawad et al. investigated the effect of hemodialysis on total PSA levels in patients with chronic renal failure, reporting insignificantly higher levels of tPSA in patients undergoing hemodialysis compared to healthy individuals. Moreover, patients with different durations of hemodialysis showed varying levels of tPSA, with lower tPSA levels in those with longer hemodialysis durations. These changes were also not significant (11). Similarly, S. Al-Janabi examined the role of PSA as a diagnostic tool for chronic renal failure in pre-dialysis patients by assessing tPSA levels in 230 CKD patients. They found normal tPSA levels (below 4 ng/mL) in 227 (98.69%) patients younger than 40 years, and elevated tPSA levels in three male patients older than 61 years with CKD affecting both kidneys. However, the elevation in tPSA was not significant in these patients (12). In both studies, tPSA levels increased as GFR decreased, consistent with the findings of our study.
According to a study by Joseph et al., there is a significant non-linear relationship between GFR and the fPSA rate, which is consistent with our findings. In their study, the fPSA rate decreased in patients with a GFR lower than 90 mL/min/1.73 m2. However, the decrease in fPSA levels was not significant in those with a GFR higher than 90 mL/min/1.73 m2. Moreover, they found a linear relationship between GFR and tPSA levels (13), which aligns with our results.
Another study by Bruun et al. investigated fPSA and cPSA levels in 101 male patients with different severities of renal failure, with a mean age of 57 years, and no history of prostate cancer or urinary tract symptoms. They compared the results with data from 5 264 healthy control men participating in the European Randomized Screening for Prostate Cancer (ERSPC). This research found that male patients with CKD had higher fPSA levels than the control group (14), consistent with our findings. However, 8 - 9% of elderly men (50 - 70 years) have elevated serum PSA levels due to benign prostatic hypertrophy (BPH) (1). Therefore, this increase may not be related to decreased renal function.
A study by Litchfield et al. demonstrated increased serum PSA levels with advancing age, reporting that 7.5% of men aged 70 - 74 years had PSA levels higher than 6.5 ng/mL, which increased to 31.4% in men older than 90 years (15). Moreover, our study showed that mean fPSA levels increased with decreasing GFR, with the difference being significant only in the age group of 60 - 69 years. Additionally, the mean fPSA level was significantly higher in 70 - 79-year-old patients with a GFR higher than 90 mL/min/1.73 m2 compared to those younger than 70 years with the same GFR range. Similarly, the mean tPSA level was significantly higher in patients aged 50 - 59 years compared to those aged 40 - 49 years. Furthermore, our study demonstrated that levels of both free and total PSA increased with decreasing serum creatinine clearance.
In another study with results differing from ours, Mahdavi et al. examined the effect of kidney transplantation on PSA, noting a significant decrease in fPSA following kidney transplantation. However, they found no relationship between tPSA and serum creatinine levels (16). Additionally, Jamil et al. reported a direct correlation between PSA and creatinine levels in individuals with prostate cancer after conducting a quantitative study of serum creatinine in these patients (17). Moreover, several studies have shown that compared to patients with low PSA, individuals with elevated PSA and advanced local or metastatic illnesses had higher creatinine levels (18). While free and total PSA increased with decreasing serum creatinine clearance in our study, serum creatinine levels can be influenced by other factors. Therefore, within its normal range, it is not a specific or sensitive marker for renal disease (19-21).
Finally, based on our results, free and total PSA increased in CKD patients with decreasing BMI. We observed a mean tPSA level of 1.55 ng/mL in patients with a BMI lower than 20 kg/m2. However, serum tPSA levels decreased to 0.79 ng/mL in patients with a BMI higher than 27 kg/m2. These findings are consistent with some previous studies reporting a reverse relationship between BMI and PSA levels (22-26) and suggest that while decreased PSA level does not reduce the chance of advanced prostate cancer, modifying PSA levels in obese men may aid in the early prediction of some invasive cases of prostate cancer (27).
One weakness of this study is its limited statistical population, which resulted in the inability to differentiate higher stages of CKD. Among the strengths of this study, we can highlight its impact in reducing prostate biopsies and subsequently minimizing possible complications.
5.1. Conclusions
The present study identified reverse relationships between free and total PSA levels with GFR and BMI. Therefore, the levels of free and total PSA increased in CKD patients with decreased GFR or BMI, indicating that renal dysfunction has a considerable effect on PSA levels and should be taken into account in PSA evaluation.