1. Background
Urinary stone disease (USD) refers to the presence of stones or crystalline material within the urinary tract. It affects approximately 12% of the global population, with a lifetime risk of formation at 8.8%, and it is more commonly observed in men. The risk of urinary stone formation increases with age, with the highest incidence occurring between the fourth and sixth decades of life (1). The incidence of urinary stone formation is nearly 0.5% per year in North America and Europe, and 50% of patients with a history of urinary stones may be at risk of developing a second stone within the next 10 years (2, 3). Various factors may contribute to the formation of urinary calculi, including socioeconomic status, dietary habits, fluid intake, and underlying medical conditions such as hypertension and diabetes mellitus (3-5).
Urinary stone disease is recognized as a risk factor for chronic kidney disease (CKD), and over time, it can progress to end-stage renal disease due to scarring resulting from hydronephrosis (6). Urinary stones can lead to life-threatening complications such as urinary tract infections, perinephric abscesses, and urosepsis. If not appropriately treated, these complications have the potential to be fatal (7). Additionally, some studies have observed an association between urinary stone formation and an increased risk of papillary renal cell carcinoma (RCC) and transitional cell carcinoma (TCC) (8-10).
There are several types of urinary stones, with calcium stones accounting for approximately 80% of cases. Hypercalciuria poses a significant risk for the formation of calcium urinary stones as it leads to the crystallization of calcium salts in association with oxalate and phosphate. Hypercalciuria may result from various factors, including increased calcium absorption in the gut, hyperparathyroidism, immobilization, and excessive sodium and animal protein consumption in the diet, either individually or in combination (11).
Vitamin D plays a crucial role in calcium and phosphorus homeostasis by mediating the intestinal absorption of calcium in the gut. Additionally, vitamin D plays a pivotal role in increasing the reabsorption of calcium and phosphorus in the kidneys, thereby modifying urinary calcium and phosphorus excretion (12-14). While hypercalciuria is established as a risk factor for USD, investigations concerning the association of serum calcium, phosphorus, and vitamin D levels with urinary stones and their characteristics are lacking. Therefore, it is imperative to optimize risk factor management due to the significance of urinary stone complications, such as chronic kidney disease (CKD) and subsequent end-stage renal disease (ESRD) resulting from hydronephrosis-induced scarring.
2. Objectives
This study aims to assess the correlation among serum calcium, phosphorus, and vitamin D levels with urinary stones.
3. Methods
3.1. Study Design and Population
This case-control study involved 180 randomly selected participants through conventional sampling. The participants consisted of 90 patients with urinary stones and 90 individuals without urinary stones who were referred to a university referral hospital in Tehran from June 2021 to June 2023. The case group included patients above 18 years old diagnosed with urinary stones based on computed tomography (CT) scan studies and referred to the urology clinic. The control group was selected from participants who had neither complaints nor a history related to urinary stones. Pregnant participants and those with underlying complications affecting calcium, phosphorus, or 25-(OH) vitamin D metabolism, such as chronic kidney diseases (CKD), end-stage renal disease (ESRD), hyperparathyroidism, kidney transplant recipients, malignancies, and malabsorption diseases, were excluded from this study. Furthermore, patients receiving diuretics, corticosteroids, calcium and vitamin D supplementation, or phosphorus chelates within 3 months of enrollment were also excluded.
The diagnosis of urinary stones was established based on imaging investigations, including ultrasonography and non-contrast abdominopelvic spiral CT scans, in patients with related complaints. Information, including demographics (sex and age) and biochemical data (serum calcium, phosphorus, 25-(OH) vitamin D), were collected from all participants in this study. After the diagnosis was established, all patients with urinary stones underwent non-contrast abdominopelvic spiral CT scans, and complementary data regarding the stones' properties, including type (struvite or non-struvite), number, side of involvement, and maximum size, were obtained.
Serum 25-(OH) vitamin D was measured using the radioimmunoassay technique for all participants in the academic referral hospital laboratory. Calcium and phosphorus levels were measured using isotope-dilution mass spectrophotometry.
3.2. Descriptions
3.2.1. 25-(OH) Vitamin D
The 25-(OH) vitamin D was classified into 4 groups based on the national institutes of health (NIH) guidelines (15) as follows: (1) Levels of 50 nmol/L (20 ng/mL) to 125 nmol/L (50 ng/mL) were considered sufficient; (2) levels below 30 nmol/L (12 ng/mL) were considered deficient; (3) levels between 50 nmol/L (20 ng/mL) and 30 nmol/L (12 ng/mL) were considered insufficient; and (4) levels above 125 nmol/L (50 ng/mL) were considered excess.
3.2.2. Calcium and Phosphorus
Normal serum levels were defined between 8.5 mg/dL to 10.5 mg/dL for calcium and 2.5 mg/dL to 4.5 mg/dL for phosphorus (16).
3.3. Ethical Consideration
All procedures involving humans were carried out following the guidelines of the Helsinki Declaration and its later editions. Written informed consent was obtained from the participants.
This study was approved by the Ethics Committee of the Research Center of Shahid Beheshti University of Medical Sciences (ethics code, IR.SBMU.MSP.REC.1399.696).
3.4. Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) 26 (IBM, New York, USA). Descriptive analysis was presented using mean and standard deviation for quantitative data, while counts and percentages were used for qualitative data. Independent sample t-test, Mann-Whitney U, and chi-square tests were used to compare the data between the 2 groups. Spearman and Pearson correlation tests were also used to assess the presence of correlations between quantitative variables. A P-value of < 0.05 was considered significant.
4. Results
The mean age of the patients was 55.07 ± 15.17 years, comprising 129 (71.6%) males. The mean age of patients with urolithiasis was 53.57 ± 15.60 years, with 63 males (70%), and 56.57 ± 14.79 years for participants without urinary stones, with 66 male individuals (73.3%).
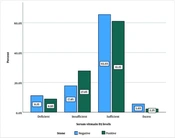
Laboratory investigations revealed mean serum calcium, phosphorus, and 25-(OH) vitamin D levels of 9.56 ± 0.65 mg/dL, 3.53 ± 0.79 mg/dL, and 25.05 ± 12.96 ng/mL overall, with 9.14 ± 0.53 mg/dL, 3.54 ± 0.73 mg/dL, and 26.26 ± 12.94 ng/mL for patients with urolithiasis, and 8.96 ± 0.75 mg/dL, 3.54 ± 0.87 mg/dL, and 27.83 ± 13.01 ng/mL for participants without urolithiasis. Sufficient vitamin D levels were observed in 64.4% of the participants, with 62.2% in the control group and 66.7% in the case group reporting sufficient vitamin D levels. Figure 1 presents the prevalence of urinary stones among participants with different levels of serum vitamin D. No significant differences were observed between the two groups regarding age, sex, and laboratory findings. Patients' demographics and laboratory findings are presented in Table 1.
| Characteristics and Levels | Total (N = 180) | US+ (N = 90) | US- (N = 90) | P-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 55.07 ± 15.14 | 53.57 ± 15.60 | 56.57 ± 14.79 | 0.066 |
| Sex | 0.620 | |||
| Male | 129 (71.6) | 63 (70) | 66 (73.3) | |
| Female | 51 (28.4) | 27 (30) | 24 (26.7) | |
| Laboratory investigations | ||||
| Serum calcium (mg/dL) | 9.56 ± 0.65 | 9.14 ± 0.53 | 8.96 ± 0.75 | 0.105 |
| Serum phosphorus (mg/dL) | 3.53 ± 0.79 | 3.54 ± 0.73 | 3.54 ± 0.87 | 0.462 |
| 25-(OH) Vitamin D (ng/mL) | 25.05 ± 12.96 | 26.26 ± 12.94 | 27.83 ± 13.01 | 0.321 |
| Vitamin D status | 0.313 | |||
| Deficient | 18 (10) | 8 (8.9) | 10 (11.1) | |
| Insufficient | 39 (21.7) | 24 (26.7) | 15 (16.7) | |
| Sufficient | 116 (64.4) | 56 (62.2) | 60 (66.7) | |
| Excess | 7 (3.9) | 2 (2.2) | 5 (5.6) |
Baseline Demographic and Laboratory Characteristics of the Participants a
Single and multiple urinary stones were observed in 57 (63.33%) and 33 patients (36.67%), respectively. Non-struvite urinary stones were observed in 79 patients (87.8%), with the largest diameter measuring 17.02 ± 7.70 mm. Unilateral stones were found in 74 patients (82.2%), with 31 cases (34.4%) on the right side and 43 cases (47.8%) on the left side, while bilateral kidney stones were seen in 16 patients (17.8%). Table 2 presents urinary stone characteristics.
| Variables and Levels | No. (%) |
|---|---|
| Number of stones | |
| Single | 57 (63.3) |
| Multiple | |
| 2 | 26 (28.9) |
| 3 | 2 (2.2) |
| 4 | 3 (3.3) |
| 5 | 1 (1.1) |
| 6 | 1 (1.1) |
| Side | |
| Unilateral | |
| Right | 31 (34.4) |
| Left | 43 (47.8) |
| Bilateral | 16 (17.8) |
| Type | |
| Struvite | 11 (12.2) |
| Non-struvite | 79 (87.8) |
Stones Characteristics Among Patients with Urinary Stones
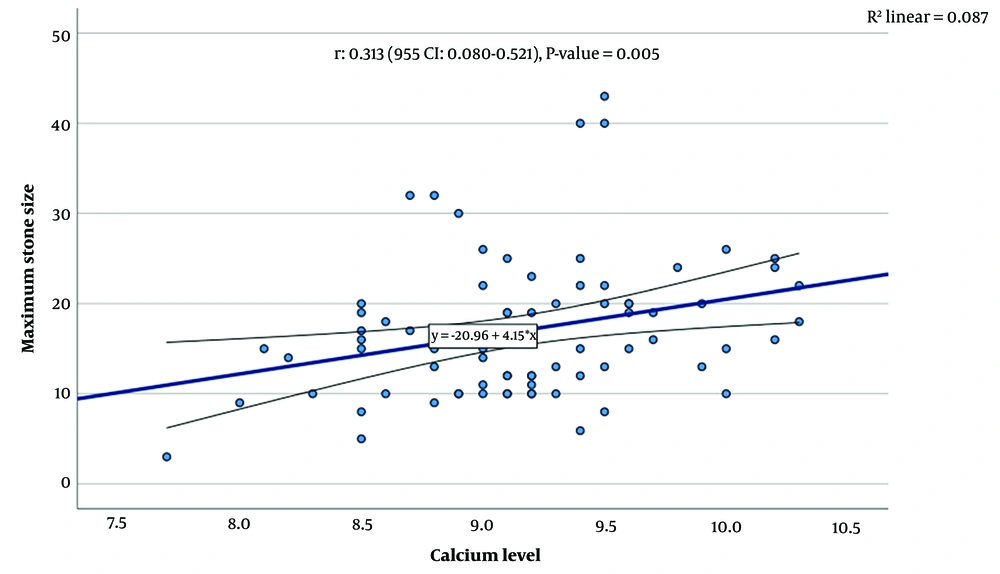
Assessing the association between stone characteristics and laboratory findings revealed a significant correlation between serum calcium levels and the maximum diameter of the kidney stone (correlation = 0.313, P-value = 0.005). No other significant associations were found between serum calcium, serum phosphorus, and serum vitamin D levels with the number of kidney stones, stone laterality, and the type of kidney stone (Figure 2).
5. Discussion
Hypercalciuria is recognized as the most common metabolic risk factor for calcium stones. Excessive urinary calcium excretion, referred to as absorptive hypercalciuria, is one of the factors responsible for this (17). Vitamin D plays a crucial role in calcium and phosphorus homeostasis by influencing the intestine and kidneys. Its primary effect is on the intestine, where it enhances calcium absorption, particularly in the duodenum. Additionally, vitamin D promotes the reabsorption of calcium and phosphorus in the kidneys (12-14, 18). Consequently, maintaining calcium and phosphorus balance and understanding vitamin D's role in this balance are crucial in the context of urinary stone formation.
However, according to this study, no associations were observed between serum calcium, phosphorus, and 25-(OH) vitamin D levels and the incidence of urinary stones. In other words, serum calcium, phosphorus, and 25-(OH) vitamin D levels did not significantly differ between participants with and without urinary stones. Furthermore, there were no significant differences in the incidence of urinary stones among individuals with deficient, insufficient, sufficient, or excess serum vitamin D levels.
Studies have produced conflicting findings concerning the role of vitamin D in urinary stone formation. Some studies found no significant differences in serum vitamin D levels between groups with and without urinary stones (19-22). Nguyen et al. reported no statistically significant association between urinary stones and serum vitamin D levels within the range of 20 to 100 ng/mL (23). Additionally, a meta-analysis revealed similar serum 25-(OH) vitamin D levels in both groups (24).
Conversely, several studies reported contrasting results (25). Ticinesi et al. and Girón-Prieto et al. separately found lower vitamin D serum levels in urinary stone formers compared to the control group and suggested that vitamin D deficiency increases the risk of urinary stone formation due to elevated iPTH levels (26, 27). Tavasoli and Taheri proposed that kidney tissue's oxidative stress and inflammation due to vitamin D deficiency could contribute to calcium oxalate stone formation in the kidney (28).
However, in another meta-analysis, Wang et al. indicated that serum vitamin D levels in kidney stone formers were significantly higher than in controls (29). In a similar study, vitamin D serum levels were significantly higher in groups with bilateral stones than in patients with unilateral stones. Similar to our investigation of the correlation between serum calcium levels and the risk of urinary stone incidence, Moudi et al. found no significant correlation between serum calcium levels and urinary stone incidence in their study (30).
In our study, we identified a significant correlation between serum calcium levels and stone size, with higher serum calcium levels associated with larger urinary stones. Nevertheless, we found no significant correlation between serum phosphorus and vitamin D levels and the size of urinary stones. Additionally, our study indicated that higher serum calcium and vitamin D levels were not risk factors for an increased number of stones in the urinary tract.
We also assessed the risk of higher serum calcium and vitamin D levels in the occurrence of bilateral urinary stones. However, we did not observe any elevated risks for the formation of bilateral kidney stones with increasing levels of calcium and vitamin D. Furthermore, there were no significant differences in serum calcium and vitamin D levels among patients with right and left kidney stones. Moreover, laboratory findings did not differ among patients with different urinary stone types.
This study represents the first attempt to evaluate the association between serum calcium, phosphorus, and vitamin D levels and stone size, number of stones, and stone types (struvite and non-struvite). However, it has several limitations. Studies investigating the impact of serum vitamin D, calcium, and phosphorus levels on urinary stone properties, such as size, location, and type, are scarce. More comprehensive studies with larger sample sizes are needed to explore the role of serum calcium and phosphorus levels in the formation of urinary stones.
In conclusion, we found that higher serum calcium levels were associated with larger urinary stone size. However, no other associations between serum vitamin D, calcium, and phosphorus levels and urinary stones were observed, including stone properties such as location and type.