1. Background
End-Stage Renal Disease (ESRD) is a severe form of kidney disease in which the patient's kidneys stop functioning permanently and irreversibly. This condition usually develops after primary kidney diseases or secondary factors such as aging, diabetes mellitus, and hypertension (1).
Since different organs of the body are affected by uremia caused by chronic kidney failure, ESRD usually manifests several signs and symptoms, including hypertension, congestive heart failure, pulmonary edema, pericarditis, skin rash, purpura, Kussmaul breathing, uremic pneumonia, ammonia breath, diarrhea, gastrointestinal bleeding, anemia, thrombocytopenia, amenorrhea, testicular atrophy, muscle cramps, and renal osteodystrophy (2-7).
The common treatment for ESRD patients is hemodialysis. Due to metabolite changes resulting from kidney malfunction, some medications, including antacids and antihypertensives, are also prescribed (8, 9).
Electrolyte balance is crucial for maintaining the proper functioning of various organs and is disrupted in ESRD patients. Some of the most prominent electrolytes are sodium, potassium, calcium, phosphorus, urea, creatinine, and parathormone hormones (PTH). However, the excretion of all the electrolytes except calcium is reduced after hemodialysis. Hence, regular assessment of these electrolytes and monitoring metabolite consumption are critical for ESRD patients (10, 11).
Although serum is used as the gold standard method for electrolyte assessment, saliva has attracted much attention in recent research. Due to the easy and non-invasive method of saliva collection and the possible close relationship between saliva and serum parameters, saliva is now considered a unique fluid for the diagnosis of ESRD (12).
2. Objectives
This study aims to investigate the correlation between the levels of sodium, potassium, phosphorus, calcium, urea, creatinine, and PTH in serum and saliva samples collected from ESRD patients undergoing hemodialysis treatment.
3. Methods
This descriptive-analytical study was performed on dialysis patients referred to Amir al-Momenin Hospital in Tehran in 2019. Purpose-based sampling was conducted among male and female volunteers aged 18 to 75 who were suffering from End-stage renal disease. End-stage renal disease was diagnosed when the glomerular filtration rate of the patient was under 15 mL/min and the last dialysis had occurred over 6 months ago (13). In addition, people under 18 years of age, recent kidney transplant recipients, smokers and alcohol users, patients with respiratory diseases and allergic reactions, and people who consumed caffeine 24 hours before the test were excluded from the study. In terms of age, gender, and body mass index, all patients were randomly selected based on the inclusion criteria (14).
After determining inclusion and exclusion criteria, PASS 15 software, considering β = 0.2, α = 0.05, and a standard deviation of 0.95, was used to achieve a sample size of 29.
The present study’s protocol followed the Declaration of Helsinki. The ethics committee of the Islamic Azad University Faculty of Dentistry, Tehran, Iran (IR.IAU.DENTAL.REC.1399.164) approved the study.
After explaining the steps to the volunteers, all eligible participants were asked to complete and sign the informed consent form. They were then asked to carefully fill out the demographic information form.
Subsequent to completing the consent and demographic forms, blood sampling was carried out before dialysis treatment for fasting patients (12 hours) by coordinating with the dialysis department. Ten cubic millimeters of ambient blood was taken from all participants and then centrifuged for 5 minutes at 3500 rpm. These samples were kept in a clot activator at -20 degrees Celsius for up to 2 months before the test. Clot activators are tubes coated with silicone, which causes the blood to clot without any other substances and allows the serum to be separated by centrifugation (14).
Saliva was collected from all the patients on the same day using the spitting method. To collect saliva, participants were asked to stop eating, drinking, and smoking one hour before the process. Next, patients were asked to allow saliva to accumulate on the floor of the mouth and then spit it into pre-weighed tubes while maintaining an upright position (15). In this method, 5 cubic millimeters of saliva was collected and placed in clean and dry paraffinized Falcon polyethylene tubes. The samples were placed on ice for 5 minutes and immediately centrifuged at 4400 RPM for 15 minutes to separate any impurities. The samples were kept at -20 degrees Celsius until testing (14).
To check serum chemical compounds including urea, creatinine, sodium, calcium, and phosphorus, a BT3000 autoanalyzer based on colorimetry was utilized. The flame photometric technique was applied to determine the amount of potassium in the serum. The Landwind Electrolyte Lwe60se device and the corresponding washing buffers were used to examine the chemical composition of saliva. To measure PTH in the serum, a Monobind kit and ELISA technique were used, and a Stat Fax 4300 chromate machine was employed for reading.
3.1. Statistical Analysis
SPSS software version 26 was used for data analysis. A paired t-test was employed to analyze the data. Moreover, Pearson's correlation coefficient was used to investigate the relationship between saliva and serum variables. In addition, values less than 0.05 and 0.01 were considered statistically significant for intergroup and intragroup comparisons, respectively.
4. Results
In this study, 29 patients (16 men and 13 women) with ESRD, with an average age of 58.27 years (minimum age of 28 and maximum age of 71 years), were investigated. The amounts of each electrolyte in both serum and saliva in ESRD patients can be observed in Table 1.
| Parameters | N | Minimum | Maximum | Mean |
|---|---|---|---|---|
| Saliva | ||||
| Na (mEq/L) | 29 | 0.90 | 5.90 | 3.9 ± 1.15 |
| K (mEq/L) | 29 | 3.10 | 7.80 | 4.63 ± 0.89 |
| Ca (mEq/L) | 29 | 0.48 | 5.30 | 2.03± 1.08 |
| P (mEq/L) | 29 | 1.60 | 7.60 | 3.47 ± 1.50 |
| Urea (mg/dl) | 29 | 31.90 | 72.80 | 50.14 ± 10.79 |
| Cr (mg/dl) | 29 | 0.61 | 1.96 | 1.21 ± 0.35 |
| PTH (pg/dL) | 29 | 0.30 | 1.80 | 1.16 ± 0.32 |
| Serum | ||||
| Na (mEq/L) | 29 | 130 | 148 | 141.34 ± 4.18 |
| K (mEq/L) | 29 | 3.50 | 6.00 | 4.51 ± 0.83 |
| Ca (mEq/L) | 29 | 7.50 | 9.20 | 8.37 ± 0.41 |
| P (mEq/L) | 29 | 3.60 | 6.20 | 4.78 ± 0.69 |
| Urea (mg/dl) | 29 | 67 | 158 | 108.66 ± 25.48 |
| Cr (mg/dl) | 29 | 3.90 | 13.20 | 8.46 ± 2.50 |
| PTH (pg/dL) | 29 | 62.00 | 2614.00 | 579.75 ± 562.23 |
4.1. Cross-Sectional Analysis of Parameters According to Their Concentration Range in Serum and Saliva
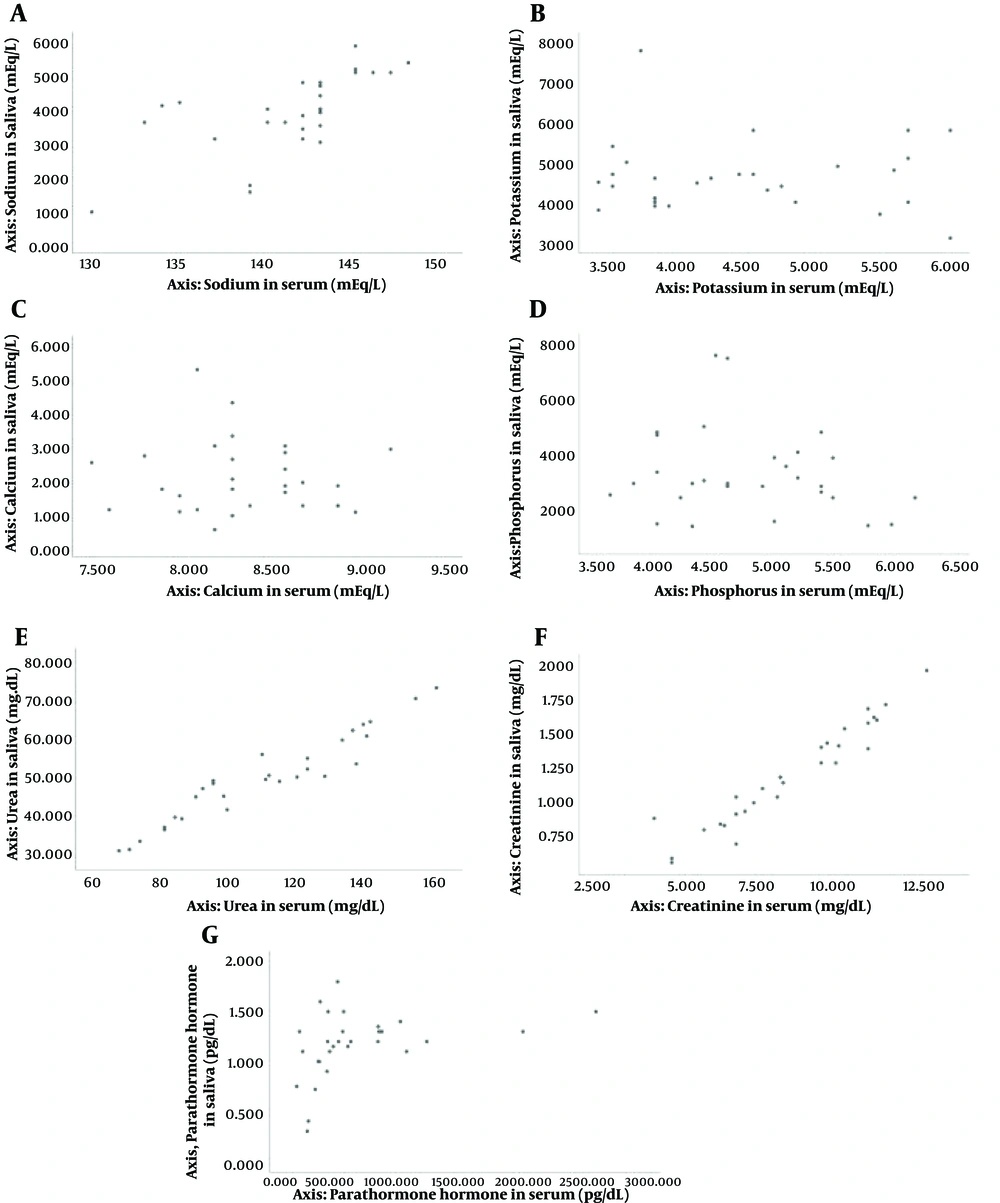
Regarding the sodium variable, all the salivary samples in the normal and less-than-normal ranges were also in the same range as the serum samples.
When the potassium variable was compared, 75% of salivary samples in the normal range were also in the same range as the serum samples. However, none of the salivary samples in the more-than-normal range matched the serum samples.
For the calcium variable, all the salivary samples in the less-than-normal range were also in the same range as the serum samples. Nonetheless, 46.2% of salivary samples in the normal range were also in the same range as the serum samples. However, none of the salivary samples in the more-than-normal range matched the serum samples.
The phosphorus variable showed different outcomes. 40.7% of salivary samples in the normal range were also in the same range as the serum samples. Moreover, 50% of the salivary samples in the more-than-normal range matched the serum samples.
When it comes to urea, creatinine, and PTH, all the salivary samples in the more-than-normal range matched the serum samples. On the other hand, 10% of PTH salivary samples in the normal range were also in the same range as the serum samples.
4.2. The Correlation of Biochemical Factors and Electrolytes Between Saliva and Serum
The results obtained from the analysis showed a significant positive correlation between the amounts of sodium, urea, and creatinine in saliva and serum (P < 0.05). This finding contrasts with the amounts of potassium, calcium, phosphorus, and PTH assessed in serum and saliva, which showed no significant correlation (P > 0.05).
Other positive correlations between different variables in saliva and serum were observed during this research. For instance, there was a significant positive correlation between salivary phosphorus and serum urea, salivary urea and serum phosphorus and creatinine, and salivary creatinine and serum phosphorus and urea (P < 0.05).
After intergroup comparison, intragroup evaluation was conducted for variables in both serum and saliva samples. In the serum group, a notable positive correlation was observed between calcium and creatinine, phosphorus and urea, phosphorus and creatinine, and urea and creatinine (P < 0.01). In the saliva group, a significant positive correlation was noted between potassium and calcium, potassium and phosphorus, potassium and PTH, calcium and PTH, phosphorus and PTH, phosphorus and urea, and urea and creatinine (P < 0.01).
A summary of the correlation between different variables in serum and saliva is shown in Table 2.
| Variables | Saliva Variables | Serum Variables | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | P | Urea | Cr | PTH | Na | K | Ca | P | Urea | Cr | PTH | |
| R | ||||||||||||||
| Na | 1 | 0.289 | 0.215 | 0.073 | -0.088 | 0.221 | -0.115 | 0.651 | 0.122 | 0.018 | 0.188 | -0.038 | 0.217 | 0.016 |
| K. | 0.289 | 1 | 0.621 | 0.409 | -0.191 | -0.011 | -0.462 | 0.204 | -0.017 | 0.009 | -0.070 | -0.118 | 0.016 | 0.096 |
| Ca | 0.215 | 0.621 | 1 | 0.338 | -0.097 | 0.220 | -0.470 | 0.228 | 0.080 | -0.080 | 0.212 | -0.040 | 0.249 | 0.066 |
| P | 0.073 | 0.409 | 0.338 | 1 | -0.451 | -0.220 | -0.380 | -0.155 | 0.099 | 0.185 | -0.191 | -0.396 | -0.212 | 0.036 |
| Urea | -0.088 | -0.191 | -0.097 | -0.451 | 1 | 0.539 | 0.264 | 0.028 | 0.119 | -0.242 | 0.520 | 0.955 | 0.455 | -0.036 |
| Cr | 0.221 | -0.011 | 0.220 | -0.220 | 0.539 | 1 | 0.182 | 0.261 | 0.282 | -0.355 | 0.413 | 0.571 | 0.959 | 0.229 |
| PTH | -0.115 | -0.462 | -0.470 | -0.38 | 0.264 | 0.182 | 1 | -0.159 | -0.197 | -0.007 | 0.161 | 0.261 | 0.139 | 0.365 |
| R | ||||||||||||||
| Na | 0.651 | 0.204 | 0.228 | -0.155 | 0.028 | 0.261 | -0.159 | 1 | 0.141 | 0.242 | 0.272 | 0.012 | 0.269 | -0.242 |
| K | 0.122 | -0.017 | 0.080 | 0.099 | 0.119 | 0.282 | -0.197 | 0.141 | 1 | -0.268 | 0.165 | 0.145 | 0.304 | -0.252 |
| Ca | 0.018 | 0.009 | -0.080 | 0.185 | -0.242 | -0.355 | -0.007 | 0.242 | -0.268 | 1 | -0.183 | -0.354 | -0.388 | -0.148 |
| P | 0.188 | -0.070 | 0.212 | -0.191 | 0.520 | 0.413 | 0.161 | 0.272 | 0.165 | -0.183 | 1 | 0.531 | 0.423 | 0.065 |
| Urea | -0.038 | -0.118 | -0.040 | -0.396 | 0.955 | 0.571 | 0.261 | 0.012 | 0.145 | -0.354 | 0.531 | 1 | 0.532 | -0.069 |
| Cr | 0.217 | 0.016 | 0.249 | -0.212 | 0.455 | 0.959 | 0.139 | 0.269 | 0.304 | -0.388 | 0.423 | 0.532 | 1 | 0.230 |
| PTH | 0.016 | 0.096 | 0.066 | 0.036 | -0.036 | 0.229 | 0.365 | -0.242 | -0.252 | -0.148 | 0.065 | -0.069 | 0.230 | 1 |
In Figure 1, the correlation between different electrolytes in serum and saliva is exhibited. The vertical axis shows the amount of the designated variable in saliva, and the horizontal axis shows the amount of the same variable in serum.
5. Discussion
The present study indicated that the amounts of some major electrolytes, including sodium, urea, and creatinine, show a positive correlation between serum and saliva. Additionally, the cross-sectional analysis of different parameters demonstrated that the normal range of the studied variables in serum is completely related to their normal range in saliva. Therefore, assessing these parameters in saliva can be utilized for monitoring ESRD patients.
Several studies have attempted to exhibit the correlation between different biomarkers in the serum and saliva of ESRD patients undergoing hemodialysis (12, 14, 16-22). However, the findings of these studies are often contrary to each other and the present study.
Regarding the sodium variable, one study by Bagalad et al. (19) compared the amount of this parameter in saliva and serum in ESRD patients, similar to the present study. Both studies exhibited a positive correlation between serum and saliva. However, another study by Seethalakshmi et al. (16) showed no significant positive correlation between serum and saliva sodium levels.
Four studies, including the present research, showed no positive correlation between serum and saliva levels of potassium, calcium, and phosphorus (12, 14, 19, 22). These outcomes differ from the findings of Seethalakshmi et al. (16), which demonstrated a positive correlation between potassium and phosphorus levels in saliva and serum.
Even though the mentioned studies showed somewhat aligned results regarding sodium, potassium, calcium, and phosphorus variables, the correlation of other parameters in serum and saliva varies across studies. For instance, a study by Rodrigues et al. (12) showed a positive correlation between serum and saliva levels of PTH, which is contrary to the findings of the present study.
Four studies illustrated a positive correlation between serum and saliva levels of creatinine, similar to the present study (17, 19-21). This finding differs from the results of the study conducted by Franco et al. (23). All the mentioned studies seem to agree on the positive correlation between serum and saliva levels of urea, which aligns with the results of this study (12, 16-22).
The reason behind the differences in the results of various studies remains unclear. Some theories include the inclusion and exclusion criteria of the studies, the existence of true ESRD patients, the methods used to collect saliva and blood, and the analyzer devices. Although all the studies compared the number of different parameters in serum and saliva, none of them performed intragroup comparisons, unlike the present study. This lack of information could question the reliability of the suggested method in the future. Therefore, more research is needed in this field.
The cross-sectional analysis of the present study indicated that the salivary concentrations of sodium, urea, and creatinine are completely dependent on their serum concentrations. This finding can be related to the reabsorption of sodium in the ducts of the salivary glands, which can explain this positive correlation. Moreover, considering that urea enters saliva through simple diffusion from plasma, it can be concluded that one of the main reasons for the strong correlation between serum and salivary concentrations of urea is this issue. On the other hand, it seems that the mechanism of creatinine entering saliva in ESRD patients is as follows: Creatinine increases in the serum under disease conditions, and increased serum creatinine in these patients creates a concentration gradient that facilitates the release of creatinine from serum to saliva. This condition is probably due to changes in the permeability of salivary gland cells. Therefore, the correlation between serum and saliva levels of sodium, urea, and creatinine seems logical.
In order to evaluate kidney function in ESRD patients and determine the timing of hemodialysis, multiple blood samples are required. The blood sampling method is invasive, and the risk of infection transmission is high. However, the saliva sampling method is non-invasive, the risk of infection transmission is low, and sampling is easy and accessible. Therefore, considering saliva sampling as an alternative method can be discussed in the future.
It should be noted that this study included only 29 patients with ESRD, which can limit the reliability of the study's outcome. Additionally, focusing solely on ESRD patients and lacking a control group consisting of individuals with healthy systemic conditions cannot precisely describe and compare electrolyte actions in the body. Therefore, future studies should consider including healthy individuals for better analysis and comparison of metabolites in the saliva and serum of both healthy and ESRD patients.
5.1. Conclusions
The results of this study exhibited a strong, meaningful relationship in the kidney function monitoring markers, including sodium, urea, and creatinine, between serum and saliva. This finding suggests it is possible to use saliva samples as a representative to evaluate kidney function monitoring factors instead of serum samples in ESRD patients. However, additional studies with a larger sample size are needed in the future to clarify this issue.