1. Background
Every year, around 60,000 new cases of malignant renal neoplasms are diagnosed, 60% of which are in the early stages. This high detection rate is largely due to the ease of access to imaging tests, particularly ultrasounds and computed tomography (CT) scans (1).
The latest consensus from the European Association of Urology (2) strongly recommends using CT of the abdomen and chest for the diagnosis and staging of renal tumors due to its high sensitivity and specificity in characterizing and detecting renal cell carcinoma.
Partial nephrectomy is the preferred surgical approach for renal neoplasms smaller than 7 cm (T1a and T1b), as it does not compromise disease-free or cancer-specific survival compared to radical nephrectomy. Additionally, partial nephrectomy can reduce the risk of renal failure and cardiovascular death (3).
With advances in surgical techniques, particularly following the widespread adoption of robotic platforms, partial nephrectomies are now being performed on patients with more complex kidney tumors (4).
To facilitate comparison between kidney tumor cases in scientific studies and assist in therapeutic decision-making, the radius, extension, nearness, anterior/posterior, and lines (R.E.N.A.L.) score, also known as the nephrometric score, was developed. This score characterizes tumor complexity based on the anatomical features of the neoplasm and aids surgeons in their treatment planning (5).
In surgery, having detailed information about the anatomy and identifying areas at the greatest risk of injury is crucial for those performing partial nephrectomies. Three-dimensional (3D) models of preoperative CT scans represent the future for accurately locating the tumor and determining the optimal surgical strategy for tumor excision and renal parenchyma preservation (6).
Currently, there are few applications available in major online stores that deal specifically with tomographic images and provide 3D virtual models in urology. None of these applications calculate the R.E.N.A.L. score to enhance surgical planning.
2. Objectives
The objective of this article is to present an application designed for medical professionals that allows for viewing renal images in 3D with the R.E.N.A.L. score based on preoperative CT or magnetic resonance imaging (MRI) exams.
3. Methods
3.1. Type of study
This is a descriptive study focused on the development of a mobile application and did not involve human subjects or animals, and therefore did not require submission or approval by an ethics committee.
3.2. Name of the Application
The application was named NeoRenal. This name was chosen for its intuitive text, aligning with the application's objective. It combines the syllables from the term “neoplasia” with the word “renal,” which pertains to the kidney.
3.3. Front-end
The entire front-end of the application was created using the Figma® website, a platform that provides tools for designing, creating, editing, and exporting ideas with the capability for collaborative creation.
3.4. Application Development
The Thunkable® platform was selected by the author to develop the back end of the application because it uses a simpler language called information blocks coding (low-code and no-code) and provides native applications for android (Google® operating system), iOS (Apple® operating system), and web (for viewing in browsers). Thunkable® is a private company based in San Francisco, California, United States, and was founded in 2015 by students from Massachusetts Institute of Technology (MIT).
3.5. 3D Reconstructions
This application has no diagnostic purpose. Therefore, the responsibility lies with the doctor, who has already used CT or MRI examinations of the abdomen with contrast to diagnose the patient's renal neoplasia, to send or share the files in DICOM format (.dcm) or the DVD with the recorded images. The 3D reconstruction is then carried out within a specified period. This reconstruction is exported to a 3D image management and visualization platform, and finally, a 3D viewer web link is created to be incorporated into the application through the web viewer function.
3.6. Database
The Firebase platform was used as a database for registering, creating, and authenticating users, as well as for storing information in the cloud (firebase.google.com). Managed by Google®, this platform enables the creation, editing, deletion, and storage of data for each application user. Only the application developers and the specific responsible user, through the registered information, have access to this data. In other words, the information registered by a user is not visible or exposed to third-party users.
4. Results
The “NeoRenal” application consists of a total of ten pages written in Portuguese, all of which were created and edited on the Thunkable platform. Each page serves a specific purpose and follows a logical order to ensure the best user experience, with considerable complexity in assembling the blocks, as highlighted below (Figure 1).
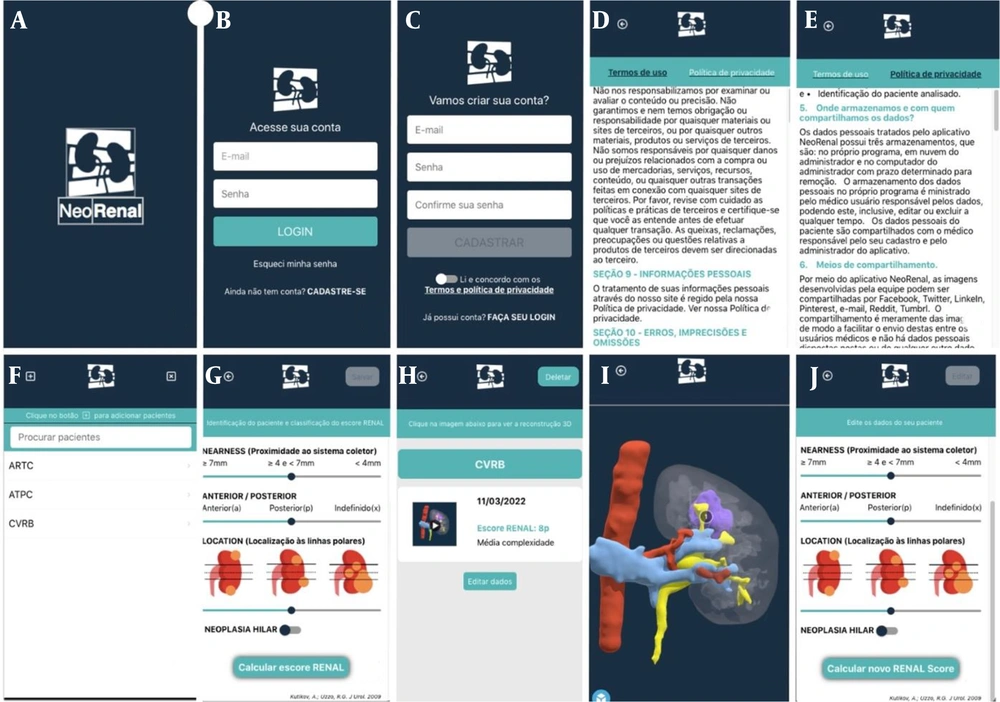
On the first screen of the NeoRenal application, users are welcomed with the application’s logo and name. This screen, programmed to display for just 3 seconds, then automatically directs users to the next screen (Figure 1A).
The second screen is for user account access. It displays the application logo and the phrase “access your account” in the authors' native language. Users can log in using their registered email and password, which must be entered into the designated text fields. After completing these fields, users must click the “LOGIN” button to proceed to the next screen. Additionally, this screen provides an option to reset the password by clicking on “I forgot my password,” which triggers an email to reset the password. First-time users can register by clicking on the “REGISTER” option. This screen also offers the option to auto-fill the user’s data on subsequent logins, streamlining the login process (Figure 1B).
The third screen allows first-time users to create an account by entering and confirming their email and password. After providing the required information, users must read and agree to the terms and privacy policies to activate the “REGISTER” button for completing registration. Existing users can return to the previous screen to log in (Figure 1C).
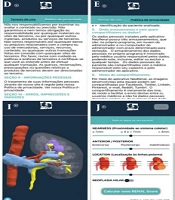
The fourth screen presents all items in the terms of use for the NeoRenal application, which must be reviewed and accepted to proceed with registration. Users can toggle between the terms of use and the privacy policy and return to the registration page to accept and continue (Figure 1D).
The fifth screen details the privacy policy of the NeoRenal application, also required for registration after approval. Users can switch between the privacy policy and the terms of use and return to the registration page to accept and continue (Figure 1E).
Screen number six is the main user interface displaying the data registered by the user. It includes a “+” button in the top left corner to add a patient's case, an “x” button in the top right corner to log out or exit the application, a text area to search for a registered patient’s name, and a list of patients registered by the user. Each user has their own list based on their individual registrations. Users only have access to their own data added to the application. This information is stored in the Firebase database and is visible only to the user and the technology team of the NeoRenal application. If this is the user's first access or if no patients have been registered yet, the search option will not appear, and an alert stating “you do not have registered patients yet” will appear in Portuguese. If the search for a patient’s name yields no results, an alert saying “patient not found” will also appear in Portuguese. Additionally, when the user clicks the “+” button to add a patient, an alert with the question “is the main lesion solid?” will appear in Portuguese, with options for a positive or negative response (Figure 1F).
The user can access the seventh screen after clicking the “+” button to add a patient or case. This screen features an arrow button in the top left corner to return to the previous screen and a “save” button in the top right corner. It is designed to identify the patient and classify the R.E.N.A.L. score. After marking the tumor characteristics using the sliders for each score, the user must click the “calculate R.E.N.A.L. score” button. Once clicked, the score results and the degree of tumor complexity will be displayed according to the score. After this, the “save” button becomes enabled (Figure 1G).
Screen number eight displays the result of clicking on the name or initial of a registered patient, which appears in the menu on the sixth screen. The data on this screen is fully customizable by the user based on the information entered for each case of renal neoplasia. In the upper left corner, there is a “back” arrow button, and in the upper right corner, a “delete” button to remove the registered case. Below, there is a text instruction in Portuguese saying “click on the image below to see the 3D reconstruction.” If the 3D reconstruction is not yet available, this instruction will alert the user. The screen also features a green band with the patient's name or initials and a white panel displaying an image of the 3D model, the date of case registration by the user, and the R.E.N.A.L. score (Figure 1H).
On this penultimate screen, the user can view and study the 3D renal neoplasia model of their specific patient directly through the application. In the upper left corner, there is an arrow button to return to the eighth screen, and below it, the interactive three-dimensional viewer allows the user to add and hide each structure of interest within the web viewer attached to the application (Figure 1I).
The tenth and final screen of the application was created to allow users to edit data they have registered if they made a mistake, without needing to delete and restart the registration process. This screen is very similar to the seventh screen with a few exceptions. Upon entering this screen, the user is asked again about the characteristic of the lesion (solid or cystic). If the lesion is cystic, a slider is also available to perform the Bosniak classification. The screen includes an “edit” button in the top right corner and a “calculate new R.E.N.A.L. Score” button at the bottom. This button allows the user to check whether the lesion is hilar and to add the “h” to the R.E.N.A.L. score. Notably, when entering this screen, the settings for each score are displayed as they were previously calculated and registered (Figure 1J).
A video of the mobile application functioning with all the screens can be seen in Appendix 1.
5. Discussion
Companies classified as healthcare startups are increasing exponentially in developed countries and have been the focus of rising investment and promotion since 2009. Healthtechs, which emphasize programming, artificial intelligence, data science, virtual reality, and augmented reality, are also of interest in business-sharing environments known as hubs. The United States is the leader in this health technology sector, representing 75% of the global market with investments of around six billion dollars in 2016 (7).
Health-related applications are available in virtual stores for the major operating systems; however, only a small minority have been validated by professionals and specialists, making it difficult to determine which ones yield positive results for patients. In 2017, it was estimated that more than 375 thousand healthcare applications were available in online stores, and between 56% and 92% of healthcare professionals used smartphone applications in their daily practice (8).
In 2015, of the 150 applications found specifically in urology, only 33% were developed in conjunction with the urological scientific community, highlighting the need for regulation and suggesting the creation of a quality seal (9).
This work demonstrates that specialist healthcare professionals can now develop applications using new low-code/no-code platforms with minimal programming knowledge.
To standardize reports and radiological characteristics of renal neoplasms, the nephrometric score, also known as the R.E.N.A.L. score, was devised. This reproducible score quantifies and characterizes the tumor based on its size; exophytic or endophytic relationship; proximity to the collection system; anterior, posterior, or hilar location; and relationship to the renal polar lines (5). A recent meta-analysis of 20 studies involving 4,717 patients concluded that the R.E.N.A.L. score is an efficient tool for determining surgical strategy and predicting complications in partial nephrectomies, with a higher renal complexity score correlating with a greater overall incidence of complications (10).
One of the innovations of our application is the availability of the R.E.N.A.L. score calculator to users after registering the patient's name. This score, along with the degree of tumor complexity (low, medium, or high), is directly accessible in the interactive 3D viewer of reconstructed kidney images.
Interactive 3D virtual models in urology are used worldwide across various platforms, with few options available in Brazil. The main easily accessible platforms and/or applications in our country offering three-dimensional models for urologists include: DocDo, a multiplatform application from the Brazilian-based company InfiniBrains; Surgiprint, a platform from the Belgium-based company of the same name; and Innersight3D (https://www.innersightlabs.com/), a platform from the UK-based company Innersightlabs. Other national platforms exist but are created and made available only to specific groups of urologists at certain hospitals or universities.
International platforms and applications offering three-dimensional models for urologists that are not available in Brazil include: Ceevra, a multiplatform application from the US-based company of the same name; Medics3D, a multiplatform application from the Italian company Medics; Cella, a multiplatform application from the Spanish company Cella Medical Solutions; VP Planning, a multiplatform application from the France-based company Visible Patient; and Mirai 3D, a platform from the Argentine company of the same name.
The NeoRenal application, which is the focus of this article, will provide an additional option for surgeons seeking to optimize their surgical planning for nephrectomies. It offers the capability to view these interactive 3D models, with the added advantage of having been developed by specialists and incorporating the R.E.N.A.L. score calculation. This allows surgeons to view their patients' three-dimensional reconstructions, organized with the renal complexity score.
A meta-analysis encompassing 10 studies with a total of 897 patients with solid organ neoplasms undergoing preoperative 3D surgical planning found that this technology can reduce operative time and blood loss without affecting length of stay or postoperative adverse events (11). Of these 10 articles, five focused on the three-dimensional planning of cases with renal neoplasia. This highlights the frequent use of this technology for patients undergoing nephrectomy and the substantial number of scientific articles published on this topic in urology worldwide. This field is of great importance and has an underexplored market due to the limited number of platforms and applications that can quickly and interactively provide 3D virtual models of the organ.
In another prospective study aimed at evaluating the impact of 3D reconstructions on arterial clamping during partial nephrectomies, evidence showed that, when the 3D model is available, the intraoperative use of selective clamping is more common (65.6% versus 15.6%, P < 0.001), without increasing blood loss or perioperative complications (12).
Approaching the renal vessels during partial nephrectomy is crucial for the urologist. Three-dimensional models enhance surgical planning at this stage, potentially reducing ischemia time to zero (selective or super-selective clamping) and significantly minimizing operative time.
5.1. Conclusions
The development of the application for use by urologists, designed to view 3D renal images with the R.E.N.A.L. score for patients with renal neoplasms based on preoperative CT or MRI exams, has been successfully completed.
Through our application, we aim to enhance scientific evidence regarding this technology, providing all urologists dealing with complex kidney cancer cases easy access to three-dimensional reconstructions and helping to reduce the morbidity associated with partial nephrectomies.
In addition to the R.E.N.A.L. score, our surgical planning 3D models may help reduce renal vessel dissection time, optimize renal ischemia time, and facilitate tumor resection.