1. Background
Zinc is one of the essential trace elements in our body, primarily stored in the liver, bones, and muscles. Zinc is involved in many functions, such as oxygen transportation, synthesis of nucleic acids, gastrointestinal absorption of other elements, and metabolism of bone, proteins, complex carbohydrates, and lipids (1). Zinc deficiency is associated with signs and symptoms, including impaired immunity, delayed wound healing, dermatitis, growth retardation, and nervousness (2).
End-stage renal disease (ESRD) is a significant challenge for developing countries. The most common causes of ESRD are diabetes mellitus (DM) and hypertension (HTN) (3). End-stage renal disease has various complications and may lead to renal replacement therapy (RRT), which can affect the patients' quality of life. Renal replacement therapy includes kidney transplantation (KT), peritoneal dialysis (PD), and hemodialysis (HD). Dialysis patients generally have a lower quality of life than the general population (4).
Zinc deficiency is a common finding among ESRD and dialysis patients. The prevalence of low serum zinc levels is estimated to be about 40% to 78% among ESRD patients (5). The effect of zinc deficiency in dialysis patients is much greater than in the general population (6). Serum zinc levels are independent predictors of hospitalization due to infection and general mortality in dialysis patients. Zinc deficiency also contributes to malnutrition in dialysis patients (7). It has been observed that low plasma zinc levels are associated with a higher depression rate among ESRD patients (8). Zinc supplementation has been found to be beneficial for sexual dysfunction, reducing cardiovascular risk, and improving immunity (9, 10).
2. Objectives
Therefore, zinc plays an essential role in the quality of life of ESRD and dialysis patients. Our study aimed to evaluate the serum level of zinc and its correlation with the quality-of-life score in hemodialysis and peritoneal dialysis patients.
3. Methods
Our study was a cross-sectional study conducted in 2018 at two different dialysis centers, Khorshid and Al-Zahra hospitals, in Isfahan, Iran. Inclusion criteria were age over 18 years, patients undergoing dialysis for at least three months, and agreement to participate in the study. Exclusion criteria included death and kidney transplantation. All hemodialysis and peritoneal dialysis patients who met the criteria and were referred to the dialysis centers were included in this study. After interviewing the patients and explaining the methods and purposes of this study, written informed consent was obtained.
Patient information and clinical data were collected and recorded in pre-made checklists. This information included age, gender, education, occupation, medical history, the main cause of renal failure, type of dialysis, dialysis duration, serum zinc level, and measurement of dialysis efficacy.
Patients’ serum zinc levels were measured by colorimeter for photometric spot measurement in their blood exams. We also assessed the quality of life using the SF-36 questionnaire. The SF-36 questionnaire includes eight subdomains: Physical functioning (PF), role limitations due to physical health (RP), pain (P), general health (GH), energy (E), social functioning (SF), role limitations due to emotional problems (RE), and emotional well-being (EW). Each domain is scored from 0 to 100, with higher scores indicating a better quality of life (11, 12). These eight domains can be summarized into two values: The Physical Component Summary (PCS) and the Mental Component Summary (MCS) scores. PCS comprises four scales: PF, RP, P, and GH. MCS comprises four scales: E, SF, RE, and EW.
Both zinc level and SF-36 questionnaires were administered at the same time the patients were referred for dialysis. For measurement of the efficacy of dialysis, we used KT/V. In the KT/V formula, K represents clearance, calculated with the urea levels before and after dialysis; T represents dialysis time; and V represents the urea distribution volume. A higher KT/V number indicates more effective dialysis (13).
The results are expressed as mean and standard deviation ± SD for quantitative variables and percentages for qualitative variables. The statistical package (SPSS 22) was used to analyze the data. The Spearman correlation test was used to assess the correlation between serum zinc level and PCS-MCS scores in general and in different subgroups. Independent sample t-tests compared zinc levels, PCS, MCS, and KT/V between different dialysis methods. For testing the differences, P-values less than 0.05 were considered significant. This study was approved by the regional bioethics committee of Isfahan University of Medical Sciences No. IR.MUI.RESEARCH.REC.1397.194.
4. Results
Inclusion criteria were met by 150 patients: 75 in the hemodialysis group and 75 in the peritoneal dialysis group. The mean age was 59.4 years (SD = 14.7), and 80 (53.3%) of the participants were male.
Table 1 shows the characteristics of the participants in the PD and HD groups. The two groups significantly differed in terms of duration of dialysis, education, and cause of ESRD. However, there was no significant difference in age, sex distribution, and presence of cardiac disease between the two groups.
| Variables | Hemodialysis Group | Peritoneal Dialysis Group | P-Value |
|---|---|---|---|
| Patients, n (male) | 75 (49.3) | 75 (57.3) | 0.8 b |
| Age, y | 59.16 ± 15.49 | 59.64 ± 14.08 | 0.41 c |
| Duration of dialysis (y) | 4.21 ± 5.09 | 2.40 ± 1.95 | 0.005 b |
| Education | 0.001 c | ||
| Illiterate | 20 (26.7) | 15 (20.0) | |
| Elementary | 10 (13.3) | 29 (38.7) | |
| High School and Diploma | 29 (38.7) | 26 (34.7) | |
| Academic | 16 (21.3) | 5 (6.7) | |
| Cardiac disease | 44 (58.7) | 36 (48.0) | 0.45 c |
| Cause of ESRD | 0.001 c | ||
| Diabetes Mellites | 36 (48.0) | 56 (74.7) | |
| Hypertension | 19 (25.3) | 13 (17.3) | |
| Other | 20 (26.7) | 6 (8.0) |
a Values are expressed as No. (%) or Mean ± SD.
b Independent samples test.
c Chi-square tests.
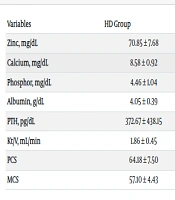
Table 2 compares laboratory data and the Physical (PCS) and Mental (MCS) Component Summary scores between the PD and HD groups. Serum zinc levels and PCS and MCS scores were significantly lower in the HD group than in the PD group. In contrast, calcium, albumin, and PTH levels were significantly higher in the HD group than in the PD group. There was no significant difference in phosphorus levels and KT/V between the two groups.
| Variables | HD Group | PD Group | P-Value b |
|---|---|---|---|
| Zinc, mg/dL | 70.85 ± 7.68 | 75.04 ± 13.54 | 0.021 |
| Calcium, mg/dL | 8.58 ± 0.92 | 8.16 ± 0.73 | 0.002 |
| Phosphor, mg/dL | 4.46 ± 1.04 | 4.60 ± 1.01 | 0.41 |
| Albumin, g/dL | 4.05 ± 0.39 | 3.56 ± 0.48 | 0.000 |
| PTH, pg/dL | 372.67 ± 438.15 | 202.79 ± 163.29 | 0.002 |
| Kt/V, mL/min | 1.86 ± 0.45 | 1.90 ± 0.51 | 0.65 |
| PCS | 64.18 ± 7.50 | 68.31 ± 8.90 | 0.003 |
| MCS | 57.10 ± 4.43 | 65.22 ± 5.64 | 0.000 |
Abbreviations: PTH, parathormone; PCS, physical Component Summary scores; MCS, Menta component summary scores.
a Values are expressed as Mean ± SD.
b P-value > 0.05 is significant.
Table 3 shows the correlation of study variables with PCS and MCS scores in patients on peritoneal dialysis and those on hemodialysis. In the HD group, PCS and MCS scores were significantly correlated, while this correlation was not observed in the PD group. Additionally, there was a positive correlation between zinc level and PCS and MCS scores in the HD group but not in the PD group. In the PD group, only age negatively correlated with PCS score, with no other significant correlations in terms of other study variables.
| Variables | HD Group | PD Group | ||||||
|---|---|---|---|---|---|---|---|---|
| PCS | P-Value | MCS | P-Value | PCS | P-Value | MCS | P-Value | |
| PCS | 1 | - | 0.76 | 0.000 | 1 | 0.21 | 0.06 | |
| MCS | 0.76 | 0.000 | 1 | 0.216 | 0.06 | 1 | ||
| Zinc | 0.52 | 0.000 | 0.58 | 0.000 | 0.16 | 0.16 | 0.01 | 0.92 |
| Calcium | - 0.11 | 0.31 | - 0.02 | 0.83 | 0.05 | 0.65 | 0.05 | 0.62 |
| Phosphor | - 0.01 | 0.92 | - 0.07 | 0.52 | 0.08 | 0.49 | 0.15 | 0.19 |
| Albumin | - 0.12 | 0.26 | - 0.02 | 0.83 | 0.16 | 0.15 | - 0.07 | 0.52 |
| PTH | - 0.08 | 0.45 | - 0.13 | 0.26 | - 0.11 | 0.31 | - 0.02 | 0.80 |
| kt/V | - 0.09 | 0.43 | - 0.15 | 0.19 | - 0.11 | 0.32 | 0.03 | 0.78 |
| age | - 0.11 | 0.33 | - 0.02 | 0.87 | - 0.25 | 0.03 | - 0.06 | 0.59 |
Abbreviations: PTH, parathormone; PCS, physical component summary scores; MCS, menta component summary scores.
a P-value > 0.05 is significant.
Table 4 shows the predictors of PCS and MCS scores in patients on peritoneal dialysis and those on hemodialysis. Multivariate analysis revealed that zinc level was an independent predictor of PCS and MCS scores in the HD group. In the PD group, age and zinc level were independent predictors of PCS score but not MCS score.
| Dialysis Method and Dependent Variables | S. Coefficients | t | Sig. | Model Summary | ANOVA | ||
|---|---|---|---|---|---|---|---|
| Beta | R2 | Adjusted R2 | F | Sig. | |||
| HD | |||||||
| PCS | 0.34 | 0.27 | 4.11 | < 0.001 | |||
| Zinc | 0.55 | 5.45 | < 0.001 | ||||
| Calcium | - 0.20 | - 1.82 | 0.07 | ||||
| Phosphor | - 0.01 | - 0.04 | 0.97 | ||||
| Albumin | - 0.12 | - 1.23 | 0.22 | ||||
| PTH | 0.07 | 0.45 | 0.65 | ||||
| kt/V | - 0.12 | - 0.84 | 0.40 | ||||
| Age | - 0.10 | - 0.99 | 0.32 | ||||
| Duration | - 0.01 | - 0.09 | 0.92 | ||||
| PD | |||||||
| PCS | 0.17 | 0.07 | 1.77 | 0.98 | |||
| Zinc | 0.25 | 2.10 | 0.03 | ||||
| Calcium | 0.01 | 0.08 | 0.93 | ||||
| Phosphor | 0.14 | 1.16 | 0.24 | ||||
| Albumin | 0.12 | 0.95 | 0.34 | ||||
| PTH | - 0.19 | - 1.65 | 0.10 | ||||
| kt/V | - 0.13 | - 1.13 | 0.25 | ||||
| Age | - 0.27 | - 2.34 | 0.02 | ||||
| Duration | - 0.02 | - 0.25 | 0.80 | ||||
| HD | |||||||
| MCS | 0.38 | 0.31 | 5.19 | 0.001 | |||
| Zinc | 0.61 | 6.14 | < 0.001 | ||||
| Calcium | - 0.14 | - 1.27 | 0.21 | ||||
| Phosphor | - 0.06 | - 0.64 | 0.52 | ||||
| Albumin | - 0.03 | - 0.33 | 0.74 | ||||
| PTH | 0.09 | 0.62 | 0.54 | ||||
| kt/V | - 0.17 | - 1.26 | 0.21 | ||||
| Age | - 0.04 | - 0.38 | 0.70 | ||||
| Duration | - 0.11 | - 1.02 | 0.31 | ||||
| PD | |||||||
| MCS | 0.05 | 0.05 | 0.50 | 0.81 | |||
| Zinc | 0.06 | 0.45 | 0.65 | ||||
| Calcium | 0.14 | 1.05 | 0.29 | ||||
| Phosphor | 0.19 | 1.51 | 0.13 | ||||
| Albumin | - 0.13 | - 0.94 | 0.35 | ||||
| PTH | - 0.06 | - 0.48 | 0.63 | ||||
| kt/V | 0.06 | 0.50 | 0.62 | ||||
| Age | - 0.09 | - 0.748 | 0.457 | ||||
| Duration | - 0.01 | - 0.046 | 0.963 | ||||
Abbreviations: HD, hemodialysis; PD, peritoneal dialysis; PTH, Parathormone; PCS, Physical Component Summary scores; MCS, Menta Component Summary scores.
5. Discussion
Since the quality of life of kidney failure patients can be a predictor of mortality and hospitalization, it is necessary to examine the quality of life, identify modifiable factors, and take necessary measures to improve the living conditions of these patients.
The results of the present study showed that HD patients had a poorer quality of life compared to PD patients, which was consistent with the findings of Kalantari et al. and Amirkhani et al. in Iran (14, 15). However, de Abreu et al. in Brazil found that PD patients had a similar quality of life to HD patients, despite being older and having more diabetic comorbidities (16). Probably, issues such as frequent visits and dependence on dialysis machines can largely explain the lower quality of life of HD patients in the present study.
Another finding of the present study was that HD patients had significantly lower zinc levels compared to PD patients. Most previous studies have reported that PD patients have similar zinc levels compared to non-dialysis chronic kidney disease patients and healthy subjects, or even higher zinc levels than HD patients (17). Decreased intake, older age, loss of urinary protein, and decreased albumin and hemoglobin probably lead to decreased plasma levels of zinc, which most likely are linked to volume expansion requiring higher glucose dialysates, greater comorbidity, and low-grade inflammation.
There was a positive correlation between zinc level and PCS and MCS scores in the HD group. Additionally, zinc level significantly predicted PCS and MCS scores in HD patients. Raimundo et al. explained that health status is compromised in about 47% of people who suffer from hypozincemia (18). In the same study, it was shown that patients who had lower serum levels of zinc, iron, and vitamin B reported a 35% decrease in quality of life (19). Numerous benefits attributed to oral zinc supplementation were cited throughout that study, and the improvement this approach brought to dialysis patients was clear. The reduction of side effects such as itching and sexual dysfunction positively affects these patients in their daily activities. In addition, other indirect benefits such as reduced systemic inflammation, reduced cardiovascular risk, and improved immune profile also indicate increased long-term quality of life as they contribute to reduced mortality in these patients (1, 20). Some studies have linked depression, a condition that affects HD patients, to zinc deficiency. Among the main causes of depression in this population are continuous hemodialysis and unemployment due to chronic kidney disease (21). However, a study by Haddadian-Khouzani et al. observed no changes in the quality of life of HD patients in the zinc group compared to the placebo group. They assessed quality of life using the Kidney Disease Quality of Life (KDQOL) instrument, which is different from the present study's method and may justify the inconsistency in results (22).
On the other hand, no correlations were found between zinc levels and PCS and MCS scores in the PD group, and zinc was only a weak predictor of PCS, but not MCS. Lack of energy, itching, muscle cramps, poor sleep, and loss of appetite are likely physical manifestations in PD patients (23); consequently, the change in PCS may be through improvement in these manifestations via zinc supplementation. In a study on seventy-nine cirrhotic patients with hepatic encephalopathy, multivariate analysis showed that zinc supplementation was significantly associated with improvement in PCS, whereas zinc supplementation was not significantly associated with changes in MCS (24).
The current study also revealed a significant relationship between age and PCS score in the PD group. In fact, with increasing age, a decrease in the quality of life was observed. Taheri et al. and Baghaie et al. also pointed out the low quality of life in elderly hemodialysis patients (25, 26). These findings are comparable to those of Parvan et al., Germin-Petrović et al., and Pakpur et al. (27-30). However, in the study conducted by Rafii et al., no relationship was found between age and quality of life (31). It is believed that with increasing age, a person's mental and physical health is affected by various factors such as chronic diseases, degeneration of organs, inability to perform personal care tasks, and the feeling of losing control over the environment, and as a result, the quality of life decreases.
Similar to findings described by other authors (32), none of the other laboratory variables was significantly associated with physical or mental quality of life. However, associations between these parameters and QoL have been indicated in some studies. For example, higher albumin, a known predictor of morbidity and mortality in dialysis populations, has been associated in many studies with better QoL (33). The reason for this association not being detected in the present study is possibly due to relatively homogeneous albumin values in this sample. In another study, the extent to which chronic kidney disease mineral bone disorder (CKD-MBD) is associated with HRQOL among incident dialysis patients was determined (34). High and low serum phosphorus and low PTH were associated with slightly poorer self-reported physical functioning. Also, in another study, the baseline level of peritoneal Kt/V urea affected the components of the quality of life after PD initiation (35).
Several limitations warrant mention in this study. First, the study was a single-center observational study with a retrospective nature. Second, serum Zn level can be influenced by patient daily life activities, dietary habits, diurnal variation, or fasting. Consequently, caution should be applied to the interpretation of our data.
5.1. Conclusions
We were able to demonstrate a positive correlation between zinc levels and improvement in the QOL in patients on dialysis (especially those on hemodialysis) in this study, so this therapy might be beneficial both mentally and physically.