1. Background
A varicocele is an unusual enlargement and twisting of the veins within the pampiniform plexus, responsible for draining blood from the testicle. Typically, varicoceles are detected after puberty, often on the left side, and are found in approximately 11 to 15 percent of adult males (1-3). Varicoceles are recognized as one of the most common treatable factors contributing to male infertility, affecting 40% of evaluated men, in contrast to 15% in the general population (4, 5). The precise way in which varicoceles impact testicular function remains unclear. The prevailing theory suggests that varicoceles can elevate testicular temperature, thereby inhibiting sperm production (6, 7). It is estimated that after undergoing varicocele repair (VR), around 60% of patients observe improvements in their seminal fluid parameters (8).
Infertility affects about 15% of couples trying to conceive globally (9). In approximately 17.1% of these cases, male factors contribute to the infertility (10). Varicocele is recognized as the most common surgically correctable cause of male infertility (11). It is not limited to infertile men; it is found in about 15% of healthy men as well (12). Moreover, varicocele is present in 35 - 44% of men with primary infertility and 45 - 81% of men with secondary infertility (13, 14). A study conducted by the World Health Organization (WHO) revealed that men with varicocele tend to have lower sperm concentration and motility compared to those without varicocele (15).
The American Urological Association and the American Society of Reproductive Medicine recommend surgical VR for clinical varicocele in non-azoospermic infertile men with abnormal seminal parameters (16). However, the term "abnormal seminal parameters" lacks a clear definition (17). Consequently, while VR is suggested for infertile men with clinical varicocele and abnormal semen characteristics, there is a lack of specific guidance on which sperm parameters should indicate the need for VR and how to assess its effectiveness. A recent global review also highlights the uncertainty surrounding whether VR is appropriate for isolated conditions like low sperm count (oligozoospermia), reduced sperm motility (asthenozoospermia), or abnormal sperm shape (teratozoospermia) (18).
Anogenital distance (AGD), the measurement from the anus to the genital area, is longer in males compared to females. In rodents, AGD is a well-established and sensitive indicator used to identify developmental issues during the critical window of masculinization (19-24). The quality of adult male semen may be influenced during fetal development, particularly within the masculinization programming period occurring between the 8th and 14th weeks of human pregnancy (23).
During this crucial period, a mother's lifestyle and exposure to chemicals that disrupt androgen function are believed to potentially harm reproductive health in adulthood. These exposures can interfere with the normal development and differentiation of the male reproductive system (21, 23, 25). Research has shown that when mothers are exposed to chemicals with anti-androgenic properties such as dioxins, phthalates, n-butylparaben, and bisphenol A (BPA), their male offspring tend to have shorter AGD (26-29). This demonstrates the significance of AGD as a relevant parameter in humans as well (22, 30).
In humans, both AGD and penile length have been reported to be shorter in individuals with conditions like hypospadias and cryptorchidism (31). Moreover, studies have found that women with higher levels of phthalates in their urine during pregnancy are more likely to have sons with shorter AGD and smaller penis length (26, 27). Other studies have shown an inverse relationship between AGD and maternal exposure to substances such as dichloro-diphenyl-dichloro-ethylene (DDE), BPA, and plasma dioxin-like compounds in maternal blood during delivery (32-34).
These disorders can potentially disrupt the growth and function of Leydig and Sertoli cells in the testicles, leading to a condition known as testicular dysgenesis syndrome (TDS) in humans (35). The interconnected symptoms of TDS, including cryptorchidism, hypospadias, testicular cancer, reduced testosterone production, impaired spermatogenesis, and recently, a shorter AGD (36), have all been linked to decreased male fertility (35, 36).
The average AGD is expected to be shorter in men with poor semen quality. Studies that have examined this relationship have found that shorter AGD is associated with reproductive issues in adulthood, including low testosterone levels, poor semen quality, and infertility (37-41). However, most of these studies have focused on infertile men, and there have been inconsistent results. For instance, in young American men, a shorter AGD was linked to worse semen quality, while no such association was observed in Spanish or Chinese men (37, 40, 42). Given that varicocele is a common treatable cause of infertility and AGD has been suggested as a potentially important factor in various studies
2. Objectives
This research aims to investigate the AGD measurements in patients seeking varicocele treatment at the Golestan Ahvaz Hospital's Urology Clinic. The study also seeks to understand whether there is a relationship between AGD and improvements in semen test results following varicocele treatment.
3. Methods
Following ethical committee approval, we conducted a prospective observational study at Golestan Hospital's Urology Clinic in Ahvaz. Our study focused on male patients with clinically evident primary varicocele and excluded those with azoospermia, secondary varicocele, or a history of orchidectomy, testicular torsion, prior malignancy, testosterone use, or chemotherapy exposure.
For all patients, we measured the distance from the back of the scrotum to the center of the anus in millimeters using a caliper while they were in the frog leg position with abducted legs. All patients underwent varicocelectomy performed by an experienced surgeon.
Patient assessments included: (1) gathering comprehensive medical histories, including infertility, sexual, surgical, and medical backgrounds; (2) conducting general examinations encompassing age, weight, height, and BMI; (3) performing local examinations, including testicular size assessed by comparison with a Prader orchidometer modified for adults and the clinical staging of varicocele; (4) measuring genital parameters (AGD) from the back of the scrotum to the edge of the anus using a caliper while patients were lying on their backs. These measurements were double-checked meticulously by the corresponding author; (5) conducting imaging studies with Doppler ultrasound of the scrotum to confirm varicocele diagnosis and measure testicle size; (6) performing laboratory examinations, covering CBC, liver and kidney function, fasting and postprandial blood sugar, and prothrombin activity. Semen analysis was performed, with patients educated on sample collection following a 2 - 7-day abstinence period. Preoperative semen analysis data were collected with at least a two-week interval between samples; (7) the surgical technique used for all patients was sub-inguinal incision microscopic varicocelectomy.
Follow-up involved assessing all patients with semen analysis six months after VR.
3.1. Statistical Analysis
For data that followed a normal distribution, we utilized the mean and standard deviation to describe the central tendency and data spread. When dealing with data that did not adhere to a normal distribution, we summarized it using the median, quartiles, and interquartile range.
To compare the means of two groups, we employed the student's t-test. For assessing the linear relationship between two continuous variables, we applied Pearson's correlation. In cases where the data did not follow a normal distribution, we substituted the Wilcoxon Signed-Rank test and Spearman's rank correlation for their normal counterparts.
3.2. Study Limitations
Since this study follows an observational design, it is important to acknowledge the possibility of selection bias and the influence of other confounding variables, including lifestyle factors (smoking, physical inactivity, daily diet), hormonal factors, and patients' psychological status, which cannot be entirely eliminated. Furthermore, it is crucial to note that the sample size may impact the study's ability to detect significant differences between the various surgical procedures. These limitations should be considered when interpreting the study's findings and drawing conclusions.
4. Results
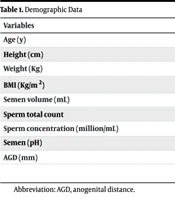
In this study, we enrolled 35 males with varicocele issues, with a mean age of 31.91 ± 5.113 years. Additional demographic characteristics and pre-surgery test results are detailed in Table 1. The values in the table are presented as mean ± standard deviation or median (interquartile range).
| Variables | Values |
|---|---|
| Age (y) | 31.91 ± 5.11 |
| Height (cm) | 175.9 ± 5.95 |
| Weight (Kg) | 80.51 ± 14.15 |
| BMI (Kg/m2) | 26.07 ± 4.52 |
| Semen volume (mL) | 3.5 ± 2.07 |
| Sperm total count | 25 (6 - 55) |
| Sperm concentration (million/mL) | 8 (1.5 - 19) |
| Semen (pH) | 7.9 (7.50 - 8.00) |
| AGD (mm) | 63.14 ± 8.2 |
Abbreviation: AGD, anogenital distance.
Following the WHO criteria, all 35 patients exhibited asthenospermia (characterized by rapid sperm motility below 32%, with 29 patients having a total fast and slow sperm motility below 40%). Five patients had hypospermia (sperm volume less than 1.5 mL), 22 patients had oligospermia (sperm concentration less than 15 million sperm per milliliter of semen), and 16 patients had alkaline semen (seminal pH equal to or higher than 8).
Subsequently, these patients underwent varicocelectomy surgery. After a 6-month post-surgery follow-up, we repeated the sperm analysis tests. Table 2 presents the findings of the sperm analysis during this 6-month follow-up period.
| Variables | Values |
|---|---|
| Semen volume (mL) | 3 (2.5 - 4.2) |
| Sperm total count | 24.44 (8.3 - 56.2) |
| Sperm concentration (million/mL) | 5.213 (2.182 - 22) |
| Semen pH | 7.734 ± 0.29 |
In the 6-month follow-up, 34 patients still exhibited asthenospermia (with 24 patients having a total fast and slow sperm motility below 40%), 3 patients remained hypospermic (semen volume less than 1.5 mL), 24 patients continued to have oligospermia (sperm concentration less than 15 million sperm per milliliter of semen), and 14 patients maintained alkaline semen (seminal pH equal to or higher than 8). Notably, two patients were diagnosed with azoospermia in the 6-month follow-up, indicating the absence of sperm in their tests. These results are summarized in Table 3, which compares sperm analysis outcomes before and after surgery.
Despite minor improvements in semen parameters 6 months after varicocelectomy surgery, our findings demonstrated no statistically significant differences in the sperm analysis results, even after considering the effect of parameters such as age, height, weight, and BMI.
| Lab Finding | Before Operation | After Operation | P-Values |
|---|---|---|---|
| Asthenospermia | |||
| < 32% (grade A) | 35 (100) | 34 (97.1) | 0.31 |
| < 40% (grade A + B) | 29 (82.9) | 24 (68/.6) | 0.1658 |
| Hypospermia | 5 (14.3) | 3 (8.6) | 0.45 |
| Oligospermia | 22 (62.9) | 24 (68.6) | 0.61 |
| Azoospermia | 0 (0) | 2 (5.71) | 0.15 |
| Alkaline Semen | 16 (45.7) | 14 (40) | 0.63 |
aValues are expressed as No (%) unless otherwise indicated.
The disparities between sperm test results before and after surgery are presented in Table 4, revealing no significant differences in the patients' test results before and after the surgical intervention.
| Variables | Paired Differences | |||
|---|---|---|---|---|
| Mean | SD | 95% CI | P-Values | |
| Semen volume | -0.0886 | 1.718 | -0.6787 to 0.5016 | 0.7622 |
| Semen pH | 0.05429 | 0.2924 | -0.04616 to 0.1547 | 0.2798 |
| Sperm concentration | 14.2691 | 85.7051 | -15.1717 to 43.7098 | 0.3316 |
| Total count | -2.1686 | 30.0647 | -12.4962 to 8.1590 | 0.6723 |
Statistical analyses were conducted to determine if there was a significant correlation between AGD and various aspects of sperm concentration before and after surgery. The results revealed no statistically significant correlation between AGD and sperm concentration before surgery (correlation coefficient = 0.014, P-value = 0.93) or between AGD and sperm concentration after surgery (correlation coefficient = -0.095, P-value = 0.58). Similarly, no significant correlation was observed between AGD and concentration differences (correlation coefficient = -0.138, P-value = 0.42) or sperm count (correlation coefficient = 0.041, P-value = 0.81) either before or after the surgical intervention.
However, it is essential to note the limitations of this analysis, including the relatively small sample size and potential confounding factors that may have influenced these findings. These limitations should be taken into account when interpreting the results.
5. Discussion
Varicocele is a common condition, affecting about one in every six men in the general population (43). Its prevalence is even higher among infertile patients, being diagnosed in approximately 19 to 41% of primary infertility cases and 80% of secondary infertility cases (43). Consequently, varicocele is often considered a contributing factor to male infertility (44).
Over the past three decades, nearly 2000 studies have examined varicocele, with roughly half investigating its impact and the effects of VR on semen parameters (45). As a result, varicocele remains one of the most debated topics in male infertility. While many international urology and reproductive societies concur on the indications for VR in cases of male infertility, such as clinically palpable varicocele and abnormal semen parameters (46), substantial uncertainties persist regarding the actual influence of VR on sperm and fertility parameters (47-49).
This study sought to explore the predictive potential of AGD concerning sperm analysis factors both before and after varicocelectomy surgery. Despite minor improvements in semen parameters six months after varicocelectomy surgery, our findings did not demonstrate statistically significant differences in sperm analysis factors before and six months after varicocelectomy surgery. This outcome contradicts the results of a substantial meta-analysis by Cannarella et al. (50). In their analysis of 351 studies, the authors suggested that all sperm analysis factors, except sperm vitality, improved after varicocelectomy repair. However, it is important to note that the reliability of their findings may be compromised due to significant heterogeneity among the results (I2 between 83 and 98%) and a noticeable publication bias in favor of data indicating a positive effect of varicocelectomy (Egger's P for all findings, except sperm morphology, was less than 0.01).
Furthermore, Shebl and Sabry conducted a prospective observational study involving 47 young men with varicocele, reporting a significant increase in sperm volume and count after six months of follow-up. Notably, the percentage of fast and slow-moving sperm, as well as sperm with progressive movement, also increased significantly, alongside improvements in sperm viability and morphology (51).
Another meta-analysis focusing on four randomized controlled trials (RCTs) investigating the impact of varicocelectomy revealed a pooled odds ratio for successful pregnancy after varicocelectomy of 2.23 [95% confidence interval (CI), 0.86 - 5.78; P = 0.091]. While this indicates a relatively superior effect of varicocelectomy compared to observation, it did not reach statistical significance. This study also considered prospective observational studies, which consistently showed that varicocelectomy was associated with a significant increase in sperm concentration, general and progressive motility, reduced seminal oxidative stress, decreased sperm DNA damage, and improved sperm ultramorphology.
It is important to acknowledge that the choice of surgical technique for VR can impact outcomes. Studies suggest that a microsurgical approach to VR results in fewer recurrences and complications compared to other techniques (52).
5.1. Conclusions
In conclusion, varicocele is a prevalent condition affecting a significant portion of the male population, particularly those experiencing infertility issues. While VR is often recommended in cases of clinically palpable varicocele and abnormal semen parameters, the impact of this surgical intervention remains a subject of debate within the scientific community.
This study aimed to investigate the predictive potential of AGD in relation to sperm analysis factors before and after varicocelectomy surgery. However, our findings did not reveal any statistically significant differences in sperm analysis factors before and six months after the surgical procedure. This outcome contrasts with the results of a substantial meta-analysis by Cannarella et al. (50), which suggested improvements in most sperm parameters following varicocelectomy. Nevertheless, the reliability of these findings was questioned due to substantial heterogeneity among the studies and potential publication bias.
Moreover, other research, including prospective observational studies, reported positive effects of varicocelectomy on various sperm parameters, seminal oxidative stress, sperm DNA damage, and sperm morphology. These studies indicated that varicocelectomy may indeed have a beneficial impact on male fertility. In fact, substantial uncertainties persist regarding the actual influence of VR on sperm and fertility parameters, and our findings suggest that infertile patients with shorter AGDs and primary varicocele have the same chance of improvement in their semen parameters after varicocelectomy.
It is worth noting that the choice of surgical technique, with a preference for microsurgical approaches, can significantly influence the outcomes of VR, leading to fewer complications and lower recurrence rates.
However, the limitations of this study, such as its relatively small sample size, need to be acknowledged. Additionally, the complex nature of varicocele and the multifactorial influences on male fertility underscore the ongoing need for comprehensive research to clarify the true effects and potential benefits of varicocelectomy. In light of the existing controversies and uncertainties, a nuanced approach, taking into account individual patient factors and a careful evaluation of the available evidence, remains crucial in the management of varicocele-associated male infertility.
Therefore, we propose designing a prospective controlled study with a larger sample size, considering more confounding factors such as lifestyle (smoking, physical inactivity, daily diet), hormonal factors, patients' psychological status, and environmental factors. Utilizing computer-assisted sperm analysis (CASA) and DFI can help overcome the uncertainties regarding the effects of varicocelectomy and AGD on the results of semen parameters and infertility in male patients.