1. Background
Prostate-specific antigen (PSA) is a protease enzyme belonging to the family of serine proteases, functioning similarly to chymotrypsin. It is also considered a member of the Kallikrein family based on its function, structure, and genetic locus. Prostate-specific antigen's primary physiological role is in liquefying the semen clot (coagulated semen) following ejaculation. Although the role of PSA in other tissues is not well understood, some researchers suggest that this protein may function as a cell growth factor (1). Prostate-specific antigen exists in plasma in various forms, with the majority bound to alpha-1-antichymotrypsin (ACT) and a smaller portion bound to alpha-2-macroglobulin (AMG). These proteins are normal plasma proteins that bind to proteases, inhibiting their function. Prostate-specific antigen is the most widely used tumor marker available for the diagnosis and follow-up of prostate cancer; however, the sensitivity and specificity of this method are not high enough to be considered an ideal tumor marker (2).
While PSA is currently regarded as a significant tumor marker for prostate cancer and a prostate tissue-specific antigen, recent reports have identified the presence of PSA in various tissues, serum, and other biological fluids, including milk, umbilical cord fluid, cerebrospinal fluid (CSF), glands around the urogenital ducts, and anal glands in both men and women, even in non-prostatic origins (3). Recent studies have shown that the progression of prostate cancer is faster, and mortality is higher in patients with low tissue PSA levels compared to those with high tissue PSA levels (4). Given the widespread use of PSA testing for prostate cancer and the development of screening programs, more than 60% of prostate cancer cases are now diagnosed in asymptomatic patients with normal digital rectal examinations and elevated PSA levels (5). Prostate cancer is the second most common cancer among men worldwide, the third most common among Iranian men, and the sixth most common cancer overall in Iran. It is often asymptomatic and frequently diagnosed at a late stage (6). However, the mortality rate from prostate cancer is lower than that of other cancers, accounting for 3.8% of all cancer-related deaths. A systematic review of biopsy studies indicates that the prevalence of prostate cancer is 5% in individuals under 30 years of age and increases to 59% in those over 79 years of age. The mortality rate of prostate cancer, however, varies by race and country of residence. The incidence of prostate cancer has been rising since the 1990s, particularly in developed countries, following the approval of PSA testing (7).
Prostate-specific antigen is a serum protease inhibitor produced exclusively by prostate tissue in response to androgen stimulation. It typically forms a complex with ACT, contributing to semen liquefaction. Serum PSA levels are usually correlated with prostate volume. Various conditions can elevate PSA levels, including benign prostatic hyperplasia (BPH), prostate cancer, prostate infection, prostate manipulation, and ejaculation. Conversely, androgen deprivation or castration, certain medications such as 5α-reductase inhibitors, and prostate removal or ablation can decrease PSA levels (8).
Prostate-specific antigen testing began in the late 1980s in the United States, leading to a significant increase in the diagnosis of prostate cancer. By 2013, more than 80% of prostate cancers detected through PSA testing were organ-confined. Although multiple isoforms of PSA, such as free and complex PSA, have been identified and utilized, the effectiveness of PSA as a screening test remains limited. Mildly elevated PSA values should prompt a repeat serum PSA test before proceeding to prostate biopsy, as a significant number of men will show normal PSA levels in subsequent tests (9).
2. Objectives
This research was conducted to investigate the relationship between PSA levels and prostate cancer in patients attending a urology clinic from 2014 to June 2023. The goal was to establish a causal relationship that could lead to improved diagnosis, the development of effective treatments, and better clinical outcomes, ultimately enhancing the management of this disease.
3. Methods
The present research was a cross-sectional analytical study.
3.1. Statistical Population, Sampling Method, and Sample Size
The statistical population consisted of all men in Ardabil province who visited a urology clinic from 2014 to June 2023, with the sampling method being full-census. The minimum and maximum ages of participants were 41 and 90 years, respectively, with a mean age of 67 ± 9.42 years.
3.2. Data Collection Method
Data collection for this study involved filling out a checklist using information extracted from patient files. The required data, such as age, prostate volume (measured by ultrasound), free and total PSA levels, urea, creatinine, and urine culture and analysis results, were gathered and recorded in the checklist. Pathological data were also obtained from the pathology center. Based on previous studies, a cut-off point of 0.18 was used to determine the free PSA-to-total PSA ratio for diagnosing prostate cancer (10). This ratio has been found to have suitable sensitivity and specificity; a ratio below 0.18 suggests a higher probability of cancer, while a ratio above 0.18 indicates a lower probability (11). Additionally, a cut-off point of 0.15 was used to determine PSA density (12), which serves as an indicator for diagnosing prostate cancer. Higher PSA density may suggest that the cancer is more aggressive or advanced (12). The Gleason score, a critical measure of prostate cancer aggressiveness, was also calculated using the pathological data (13, 14).
3.3. Inclusion and Exclusion Criteria
The inclusion criteria for this study included a history of transrectal ultrasound (TRUS)-guided prostate biopsy and an abnormal blood PSA level (above 4 ng/mL) (15). The exclusion criteria were the absence of prostate volume data in the patient file, the absence of blood PSA level results, and the absence of urine culture and analysis results.
3.4. Data Analysis Method and Statistical Analysis
SPSS version 21 software was used for data analysis. In this study, the mean and standard deviation were utilized to describe quantitative data, while frequency and percentage (presented in diagrams and tables) were used to describe qualitative data. The chi-square test was employed to assess the relationship between qualitative variables, such as the association of biopsy results with the Gleason score and PSA level variables. Additionally, the analysis of variance (ANOVA) test was used to compare age among the four groups: BPH, PIN, cancer, and normal.
3.5. Ethical Considerations
The current research was approved by the Research Ethics Committee of Ardabil University of Medical Sciences (code of ethics: IR.ARUMS.MEDICINE.REC.1402.045). Additionally, patients' privacy and confidentiality were fully maintained throughout the study.
4. Results
4.1. The Relationship Between Prostate-Specific Antigen Levels and Prostate Biopsy Results
According to Table 1, for PSA concentrations less than 10 ng/mL, the highest proportion was observed in the PIN group (46.5%), while the lowest was in the normal group (5.1%). For PSA concentrations between 10 - 50 ng/mL, the highest proportion was in the cancer group (37.7%), and the lowest was in the normal group (6.1%). For PSA concentrations over 50 ng/mL, the highest proportion was again in the cancer group (75.9%), and the lowest was in the normal group (3.4%).
| Biopsy Result | |||||
|---|---|---|---|---|---|
| PSA Values | Normal | Cancer | PIN | BPH | Total |
| Less than 10 | 5 (5.1) | 15 (15.2) | 46 (46.5) | 33 (33.3) | 99 (100) |
| 10 - 50 | 7 (6.1) | 43 (37.7) | 30 (26.3) | 34 (29.8) | 114 (100) |
| Over 50 | 1 (3.4) | 22 (75.9) | 4 (13.8) | 2 (6.9) | 29 (100) |
Abbreviations: PSA, prostate-specific antigen; PIN, prostate intraepithelial neoplasia; BPH, benign prostatic hyperplasia.
a Values are expressed as No (%).
Additionally, based on the chi-square test results, there is a significant relationship between PSA levels and biopsy results (P < 0.0001). Specifically, as PSA concentration decreases, the frequency of individuals with cancer also decreases, and conversely, as PSA concentration increases, the frequency of cancer cases increases.
4.2. The Relationship Between Age and Biopsy Results
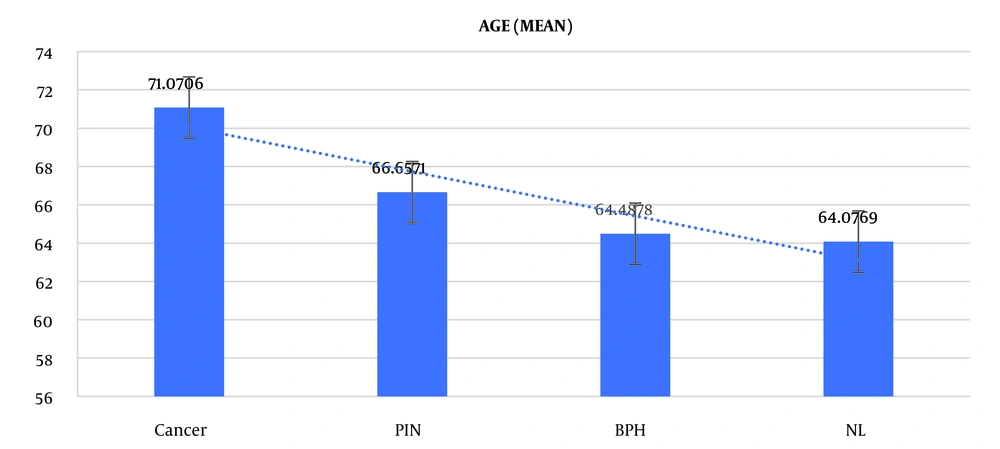
Based on the ANOVA test results in Table 2, which displays the age distribution of the investigated subjects based on biopsy results (including healthy, cancer, BPH, and PIN), a significant difference in age was observed between the different biopsy result groups (P < 0.001, F = 8.252) (Figure 1).
| Variables | Age | ||
|---|---|---|---|
| Biopsy results | Number | Mean | Standard Deviation |
| Normal | 13 | 64 | 8.9 |
| Cancer | 85 | 71 | 8.7 |
| BPH | 82 | 64.4 | 9.1 |
| PIN | 70 | 66.6 | 9.2 |
| Total | 250 | 67.3 | 9.4 |
Abbreviations: BPH, benign prostatic hyperplasia; PIN, prostate intraepithelial neoplasia.
According to the ANOVA (Tukey) post-hoc test results in Table 3, there is a significant difference in age between the cancer group and the other three groups (P < 0.05); however, no significant difference was observed between the other groups.
| Dependent Variable: Age, Tukey Post-hoc Test | |
|---|---|
| Biopsy Results | Significance |
| Healthy | |
| Cancer | 0.048 |
| BPH | 0.999 |
| PIN | 0.78 |
| Cancer | |
| Healthy | 0.048 |
| BPH | 0.0001 |
| PIN | 0.014 |
| BPH | |
| Healthy | 0.000 |
| Cancer | 0.0001 |
| PIN | 0.454 |
| PIN | |
| Healthy | 0.78 |
| Cancer | 0.014 |
| BPH | 0.454 |
abreviations: PIN, prostate intraepithelial neoplasia; BPH, benign prostatic hyperplasia.
a The mean difference is significant at the 0.05 level.
4.3. The Relationship Between Prostate-Specific Antigen Levels and the Gleason Score in Individuals with Prostate Cancer
According to the data in Table 4, the chi-square test indicates that there is no significant relationship between PSA levels and the severity of adenocarcinoma as determined by the Gleason score (P > 0.05).
| Three PSA Levels | Gleason Score | Total | ||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Less than 10 | 10 (71.4) | 2 (14.3) | 2 (14.3) | 14 |
| 10 - 50 | 22 (53.7) | 12 (29.3) | 7 (17.1) | 41 |
| Over 50 | 7 (31.8) | 9 (40.9) | 6 (27.3) | 22 |
Abbreviation: PSA, prostate-specific antigen.
aValues are expressed as No (%) unless otherwise indicated.
4.4. The Statistical Analysis of the Relationship Between Prostate Cancer and the Free Prostate-Specific Antigen-to-Total Prostate-Specific Antigen Ratio and the Prostate-Specific Antigen-to-Prostate Density
According to Table 5, the free PSA-to-total PSA ratio in this study was 0.18. The chi-square test indicated a significant relationship between prostate cancer and the free PSA-to-total PSA ratio (P < 0.05). Furthermore, according to Table 6, the cut-off value for the PSA-to-prostate volume (prostate density) ratio is 0.15. The chi-square test demonstrated a significant relationship between prostate cancer and the PSA-to-prostate volume (prostate density) ratio (P < 0.001).
| Categorization Based on the Cut-off Point of FPSA/TPSA | Biopsy | Total | P-Value | |||
|---|---|---|---|---|---|---|
| Normal | Cancer | BPH | PIN | |||
| 0.18 < | 4 (3.3) | 39 (32.5) | 38 (31.7) | 39 (32.5) | 120 | 0.001 |
| 0.18 > | 6 (8.2) | 18 (24.7) | 36 (49.3) | 13 (17.8) | 73 | |
Abbreviations: FPSA, free prostate-specific antigen; TPSA, total prostate-specific antigen; PIN, prostate intraepithelial neoplasia; BPH, benign prostatic hyperplasia.
aValues are expressed as No (%) unless otherwise indicated.
| Density in Two Categorizations in the Cut-off Point of 0.15 | Biopsy | Total | P-Value | |||
|---|---|---|---|---|---|---|
| Normal | Cancer | BPH | PIN | |||
| 0.15 < | 3 (3.7) | 9 (11.1) | 38 (46.9) | 31 (38.3) | 81 | P < 0.001 |
| 0.15 > | 10 (6.2) | 71 (44.1) | 42 (26.1) | 38 (23.6) | 161 | |
Abbreviations: FPSA, free prostate-specific antigen; TPSA, total prostate-specific antigen; PIN, prostate intraepithelial neoplasia; BPH, benign prostatic hyperplasia.
aValues are expressed as No (%) unless otherwise indicated.
4.5. The Statistical Analysis of the Relationship Between Active Urinary Infection, Prostate-Specific Antigen Levels, and Biopsy Results
In Table 7, 26% of the samples had a urinary infection at PSA levels less than 10 ng/mL, 54% at PSA levels between 10 - 50 ng/mL, and 20% at PSA levels over 50 ng/mL. The chi-square test indicated a significant relationship between PSA levels and urinary infection (P < 0.05). The incidence rate of urinary infection was 3.8% in the normal group, 36.5% in the cancer and BPH groups, and 23.1% in the PIN group (Table 8). However, the chi-square test showed no significant relationship between urinary tract infection and the different biopsy results (P > 0.05). Notably, the biopsies were performed after the completion of the urinary infection treatment course and following a negative urine culture.
| Urinary Tract Infection | Categorization of PSA Levels | Total | P-Value | ||
|---|---|---|---|---|---|
| Less Than 10 | 10 - 50 | Over 50 | |||
| Yes | 13 (26) | 27 (54) | 10 (20) | 50 | < 0.05 |
| No | 86 (44.8) | 87 (45.3) | 19 (9.9) | 192 | |
Abbreviation: PSA, prostate-specific antigen.
a Values are expressed as No (%) unless otherwise indicated.
| Urinary Tract Infection | Biopsy | Total | P-Value | |||
|---|---|---|---|---|---|---|
| Normal | Cancer | BPH | PIN | |||
| Yes | 2 (3.80) | 19 (36.50) | 19 (36.50) | 12 (23.10) | 52 | > 0.05 |
| No | 11 (5.6) | 66 (33.3) | 63 (31.80) | 58 (29.3) | 198 | |
Abbreviations: BPH, benign prostatic hyperplasia; PIN, prostate intraepithelial neoplasia.
aValues are expressed as No (%) unless otherwise indicated.
5. Discussion
The present research aimed to investigate the relationship between abnormal PSA levels and biopsy results in patients who visited a urology clinic in Ardabil from 2014 to June 2023. Overall, the data from this study indicated a direct relationship between plasma PSA concentrations and the likelihood of prostate cancer. The results also demonstrated that plasma PSA concentrations, while directly related to age, are also associated with the severity of trophic disorders such as cancer, as reflected in biopsy results.
The data indicated that as PSA concentrations increased, the prevalence of prostate cancer among the samples also increased. For instance, at PSA concentrations less than 10 ng/mL, 5% of the samples were healthy, 15.2% had cancer, 46% had BPH, and 33% had PIN. Silva et al. reported these values as 33% for cancer, 33% for BPH, and 15% for PIN, suggesting that differences in genetic and racial characteristics between the samples of the two studies could account for the discrepancies (16). On the other hand, Freitas reported the prevalence rate of BPH at this PSA level to be around 40%, which is consistent with the results of this study (17). Lazzeri et al. reported a value of 27% for BPH at this level (18). These findings suggest that PSA concentrations below 10 ng/mL remain a diagnostic challenge (19). In this regard, Merriel et al. indicated that while PSA is highly sensitive for diagnosing prostate cancer in symptomatic patients, it has low specificity, which aligns with the findings of this research (20). Prostate cancer detected via biopsy, including high-grade cancers, was not rare among men with PSA levels of 4 ng/mL or less, supporting the findings of this study that cancer incidence is possible at this concentration, although with a low probability.
Similarly, at PSA concentrations of 10 - 50 ng/mL, 6% of the samples were healthy, 37% had cancer, 26% had BPH, and 29% had PIN. PutRA et al. suggested that the incidence rate of cancer in this range is around 33.5%, which is close to the findings of this research (21). Venkatachalapathy et al. reported the prevalence rate of cancer at this level to be 50%; the discrepancy may stem from the different objectives of the two studies (22), as the mentioned research focused primarily on identifying individuals with cancer, while this research examined the entire patient population.
At PSA concentrations above 50 ng/mL, 3% of the samples were healthy, 76% had cancer, 14% had BPH, and 7% had PIN. Lojanapiwat et al. reported the prevalence of prostate cancer at PSA levels of 4.1 - 10, 10.1 - 20, 21.1 - 50, 50.1 - 100, and >100 ng/mL to be 9.3%, 55.5%, 87.5%, 98.2%, and 99.7%, respectively (23). Overall, these findings suggest that higher PSA concentrations are associated with worse biopsy outcomes. The rate of PSA expression in individuals was directly associated with the incidence of cancer, and measuring PSA levels could potentially reduce mortality rates due to prostate cancer; however, this marker may also lead to overdiagnosis in some patients. Nam et al. also found that PSA tissue expression was related to the incidence of prostate cancer; however, they emphasized that this method should be used in conjunction with radiological findings, such as magnetic resonance imaging (MRI), to increase its diagnostic value (24).
Additionally, the data show that plasma PSA levels, while directly related to age, are also directly related to the severity of trophic disorders such as cancer in biopsy results. This relationship can be interpreted in two ways. First, increasing age is associated with worse biopsy outcomes. Second, as age increases, PSA levels tend to rise, further indicating a correlation between age, PSA levels, and the severity of prostate cancer.
Liu et al. found in China that the impact of age on the occurrence and mortality of prostate cancer tends to increase with age, particularly in the elderly (25). Similarly, the findings of Vickers et al. are noteworthy because, as in our study, the prognosis of prostate trophic disorders worsened with age. Additionally, their research indicated that elevated PSA levels were more pronounced in older individuals and were associated with the occurrence of malignancies (26). In a 20-year cohort study, Pierre-Victor also demonstrated that PSA levels increase with age, which in turn elevates the likelihood of developing prostate cancer, aligning with the results of our study (27). As discussed, the incidence rate of prostate cancer becomes more prevalent among elderly men as they age, and according to the present findings, this increase is associated with worse biopsy outcomes. It is therefore plausible that as this prognosis worsens, PSA levels in the plasma of elderly individuals may show higher concentrations.
The current data also demonstrated that with a free PSA-to-total PSA ratio of 0.18, the severity of biopsy results worsened. Additionally, the data showed that a PSA-to-prostate volume ratio of 15% was associated with greater severity. The free PSA-to-total PSA ratio of 0.18 for diagnosing prostate cancer, as found in our study, is similar to the findings reported by Jansen et al., who identified this ratio between 20% and 30%, which is somewhat consistent with our research (28). Given the varying results, further research is needed to determine the exact ratio, taking into account different factors such as ethnicity. It is expected that PSA levels would be higher in men with larger prostates due to increased PSA production, while higher PSA density may indicate the presence of cancer (29). In this regard, Lopes Vendrami et al. suggested that PSA density could play a crucial role in determining the severity of prostate neoplasms (30). The association between a PSA density greater than 0.15 and a higher likelihood of prostate cancer (up to 80%) is consistent with the findings of this study. Abonyo et al. also indicated that density values above 0.15 could be related to a worse prognosis, with increasing density leading to more severe biopsy outcomes (31). Furthermore, Wang et al. showed that while higher PSA density values could indicate cancer, blood PSA levels might be falsely low (below 4 ng/mL), leading to misdiagnoses (32). Based on this evidence, these two criteria—PSA density and free PSA-to-total PSA ratio—could help improve the diagnosis of prostate cancer when used alongside PSA level measurements, though further research is necessary to fully understand these relationships.
In the present research, 13% of samples had infections at PSA levels less than 10 ng/mL, 54% had infections at levels of 10 - 50 ng/mL, and 20% had infections at levels over 50 ng/mL, and this trend was significant. At PSA levels less than 10 ng/mL, the rate of urinary infection was 11% - 15%, which aligns with our findings. This rate increased to 19% at levels over 10 ng/mL, though this difference could stem from variations in measurement methods. The incidence of urinary tract infections, particularly near the prostate gland, can cause blood PSA levels to rise significantly, though not to the same extent as in cases of prostate malignancy, which is consistent with the results obtained in this study. Additionally, it has been shown that after administering antibiotics and resolving the urinary infection, PSA levels typically return to normal, i.e., less than 4 ng/mL. At PSA levels over 10 ng/mL, the sensitivity of this criterion for detecting urinary tract infection could be over 69%, which aligns with the findings of this research. Prostate-Specific Antigen levels above 10 ng/mL have also been linked to the occurrence of inflammation and urinary tract infections after kidney transplantation, generally supporting the results obtained, although that research had different objectives than the present study (33). Based on these findings, PSA levels of 10 - 50 ng/mL appear to be associated with the likelihood of urinary tract infection. This relationship can be seen as both an opportunity and a challenge. On one hand, it could improve the diagnosis of infections in the urinary ducts. On the other hand, this overlap might lead to false diagnoses, as mentioned in some studies (34, 35).
On the one hand, the evidence indicated that the incidence rate of urinary infection was 3.8% in the normal group, 36.5% in the cancer and BPH groups, and 23.10% in the PIN group. Onyebueke et al. reported the prevalence rate of urinary tract infection among BPH patients to be 93% (36). In contrast, the prevalence rate among BPH patients was 62%, while Safwat et al. calculated this value to be 9% (37). Additionally, Tolani et al. calculated the occurrence rate of urinary tract infection among individuals with prostate cancer to be 35%, which is close to our findings (38). Heyns also reported this value to be 36% (39). Based on the aforementioned data, it appears that although the incidence of urinary tract infection in the current research showed no significant relationship with biopsy prognosis, it follows a certain trend that requires further investigation to be fully understood.
5.1. Limitations
The relationship between age and PSA density was not investigated. The relationship between confounding factors and the occurrence of prostate cancer was not explored. The effect of race on the occurrence of prostate cancer was not examined. The incompleteness of the files led to the exclusion of a large number of them from the study. The absence of computerized records for the files resulted in significant time being spent reviewing them manually.
5.2. Conclusions
Prostate cancer is now recognized as one of the leading causes of mortality in men. Various diagnostic approaches have been developed to assess its severity, each with specific advantages and disadvantages. Among these, plasma PSA level is considered an accessible and relatively cost-effective marker. Although numerous studies have been conducted to understand the trends in PSA level changes, a complete understanding of this process is still lacking, and further research is needed. The present evidence demonstrated that plasma PSA concentration is directly linked to the likelihood of prostate cancer. Additionally, the current results indicated that plasma PSA concentration, while directly related to age, is also associated with the severity of trophic disorders such as cancer, as seen in biopsy results. However, it is noteworthy that 84.8% of biopsies at PSA concentrations less than 10 ng/mL, 63% of biopsies at PSA concentrations of 10 - 50 ng/mL, and 24% of biopsies at PSA concentrations over 50 ng/mL were not cancerous, suggesting that biopsies may not be necessary for all patients. Furthermore, the data demonstrated that a PSA density greater than 0.15 ng/mL and a free PSA-to-total PSA ratio less than 0.18 are associated with an increased risk of cancer.